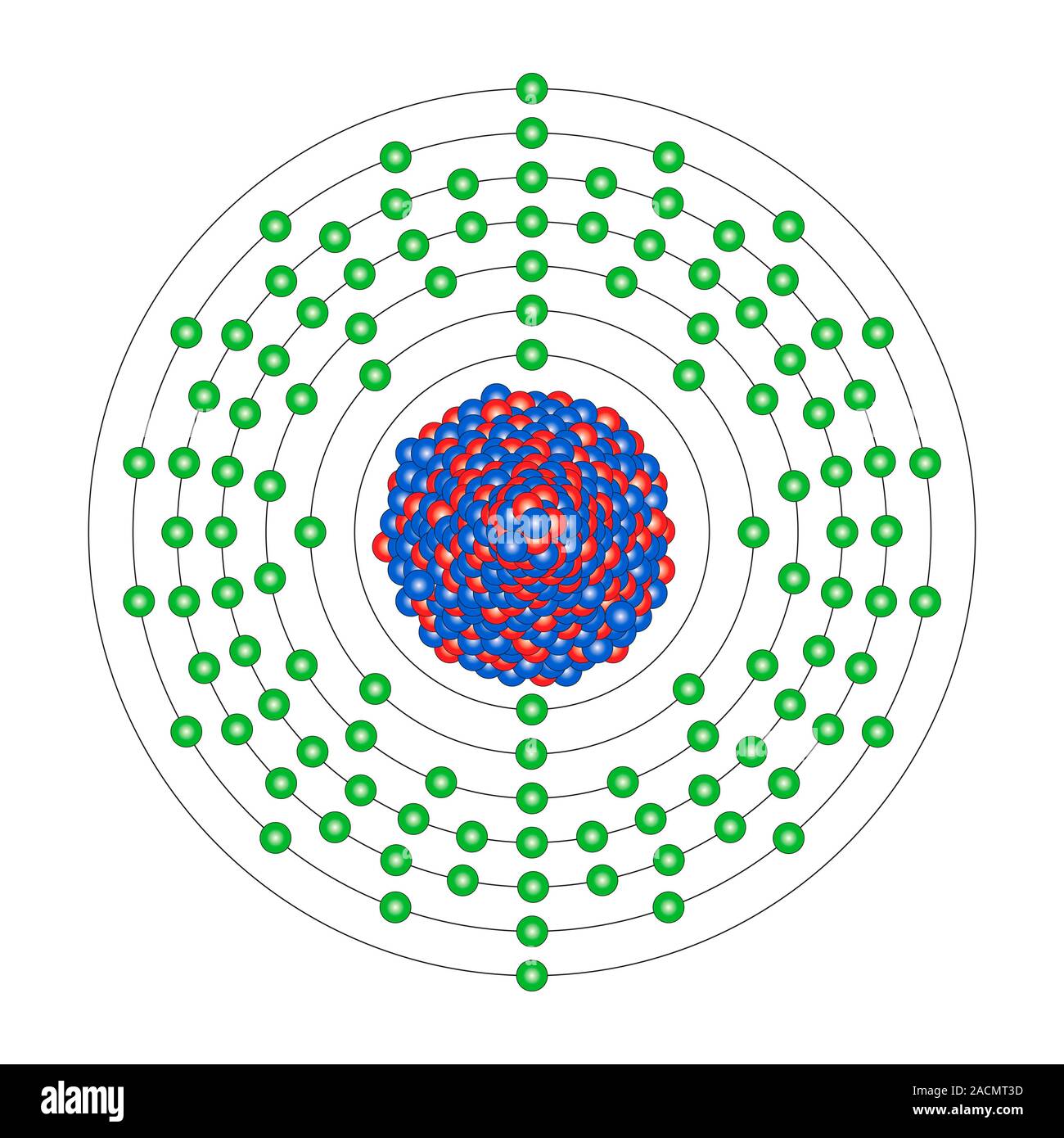
Copernicium (Cn). Diagram of the nuclear composition and electron configuration of an atom of copernicium-285 (atomic number: 112), the most stable is
RMID:Image ID:2ACMT3D
Image details
Contributor:
Science Photo Library / Alamy Stock PhotoImage ID:
2ACMT3DFile size:
60 MB (943.7 KB Compressed download)Releases:
Model - no | Property - noDo I need a release?Dimensions:
4579 x 4579 px | 38.8 x 38.8 cm | 15.3 x 15.3 inches | 300dpiDate taken:
3 May 2012Photographer:
Science Photo LibraryMore information:
Copernicium (Cn). Diagram of the nuclear composition and electron configuration of an atom of copernicium-285 (atomic number: 112), the most stable isotope of this radioactive element. The nucleus consists of 112 protons (red) and 173 neutrons (blue). 112 electrons (green) bind to the nucleus, successively occupying available electron shells (rings). Copernicium, named after Nicolaus Copernicus and first synthesised in 1996, is a transactinide and transition metal in group 12, period 7, and the d-block of the periodic table. Copernicium-285 has two forms (nuclear isomers), with half-lives of 29 seconds and 8.9 minutes.