Chemical lecture experiments . sbestos ; NH3 supply. 34. Electrolysis of ammonium hydroxide. — Ammonia isdecomposed into the elements hydrogen and nitrogen whensubjected to electrolysis. Strong ammonium hydroxide, to which some sodium chlo-ride has been added to give conductivity to the solution, ispoured into the electrolytic apparatus (Fig. 46, p. 95) anddecomposed by means of a current of from four to sixcells. Platinum electrodes must be used. In a shorttime gas will be collected at both arms of thetube in the proportion of three volumes in onearm to one volume in the other. The largervolu
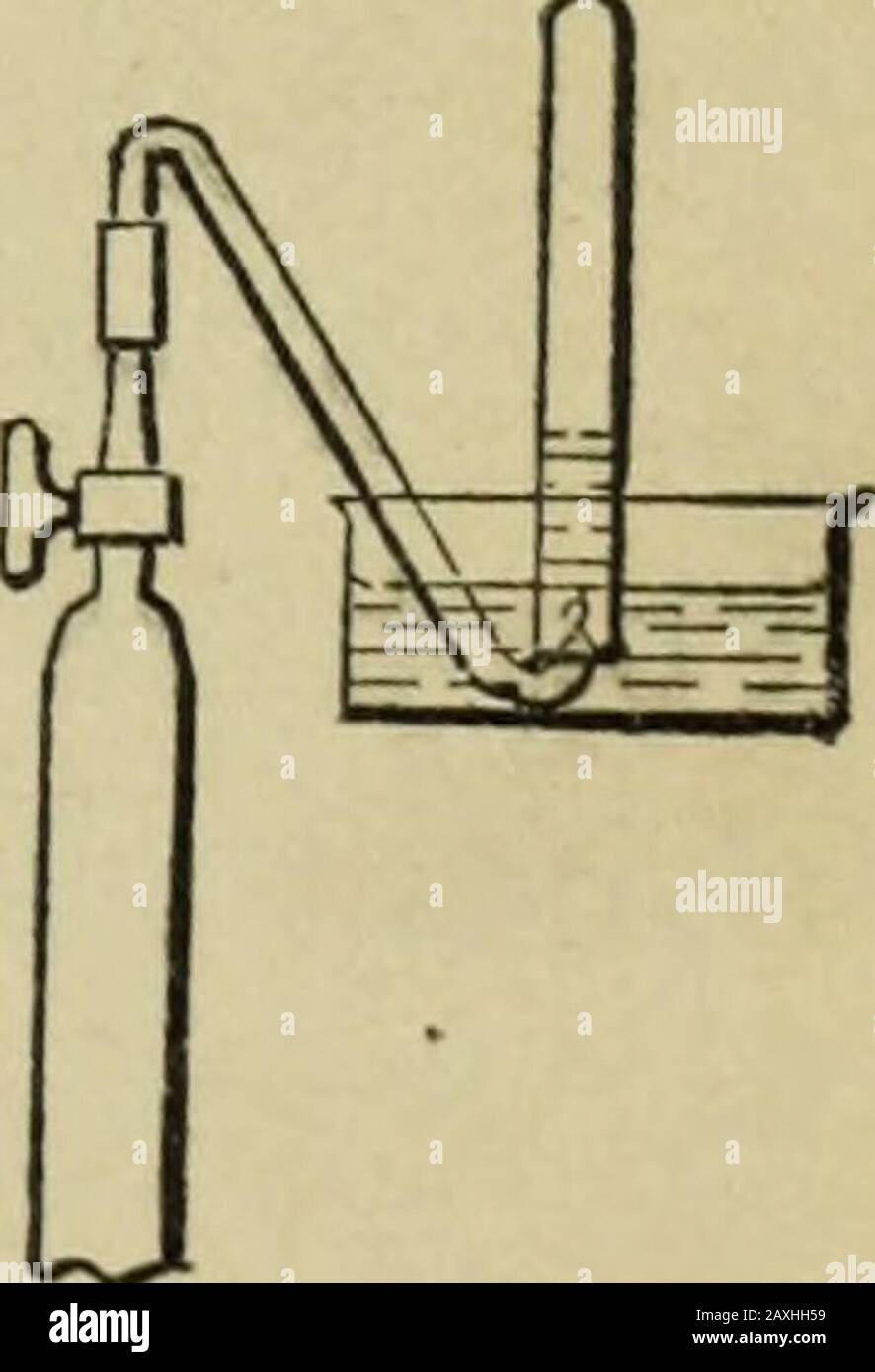
Image details
Contributor:
The Reading Room / Alamy Stock PhotoImage ID:
2AXHH59File size:
7.1 MB (132.1 KB Compressed download)Releases:
Model - no | Property - noDo I need a release?Dimensions:
1305 x 1914 px | 22.1 x 32.4 cm | 8.7 x 12.8 inches | 150dpiMore information:
This image is a public domain image, which means either that copyright has expired in the image or the copyright holder has waived their copyright. Alamy charges you a fee for access to the high resolution copy of the image.
This image could have imperfections as it’s either historical or reportage.
Chemical lecture experiments . sbestos ; NH3 supply. 34. Electrolysis of ammonium hydroxide. — Ammonia isdecomposed into the elements hydrogen and nitrogen whensubjected to electrolysis. Strong ammonium hydroxide, to which some sodium chlo-ride has been added to give conductivity to the solution, ispoured into the electrolytic apparatus (Fig. 46, p. 95) anddecomposed by means of a current of from four to sixcells. Platinum electrodes must be used. In a shorttime gas will be collected at both arms of thetube in the proportion of three volumes in onearm to one volume in the other. The largervolume of gas can be tested and shown to behydrogen by opening the gas-cock and applyinga match. The sodium chloride in the solutionwill, however, impart a yellowish color to the jrIG# §9flame. The smaller volume of gas can be trans-ferred to a test-tube in the manner shown in Fig. 89, andproved to be nitrogen by extinguishing a match. 2NH3 = N2 + 3H2. Electrolytic apparatus (Fig. 46, p. 95); battery 6 cells; con.NH4OII ; NaCl.. 202 CHEMICAL LECTURE EXPERIMENTS 35. Quantitative decomposition of ammonia by chlorine. — Chlorine acts on ammonium hydroxide with the forma-tion of hydrochloric acid and the liberation of nitrogen.Chlorine abstracts the hydrogen from ammonia, and conse-quently for every three volumes of chlorine used one volumeof nitrogen is liberated. The eudiometer (Fig. 11, p. 26) is filled over a salt solu-tion with a rapid stream of pure chlorine. When the tubeis completely filled, a well-fitting rubber stopper is crowdedinto the open end of the tube while still under the saltsolution, care being taken to enclose no liquid in the tube.A few cubic centimeters of strongest ammonium hydroxideare then allowed to flow through the funnel into the gas, though the stop-cock must be but slightly turned. As theammonia comes in contact with the chlorine, the reaction isvery vigorous and, owing to the condensation in gas volume, apartial vacuum is produced inside the tube. Th