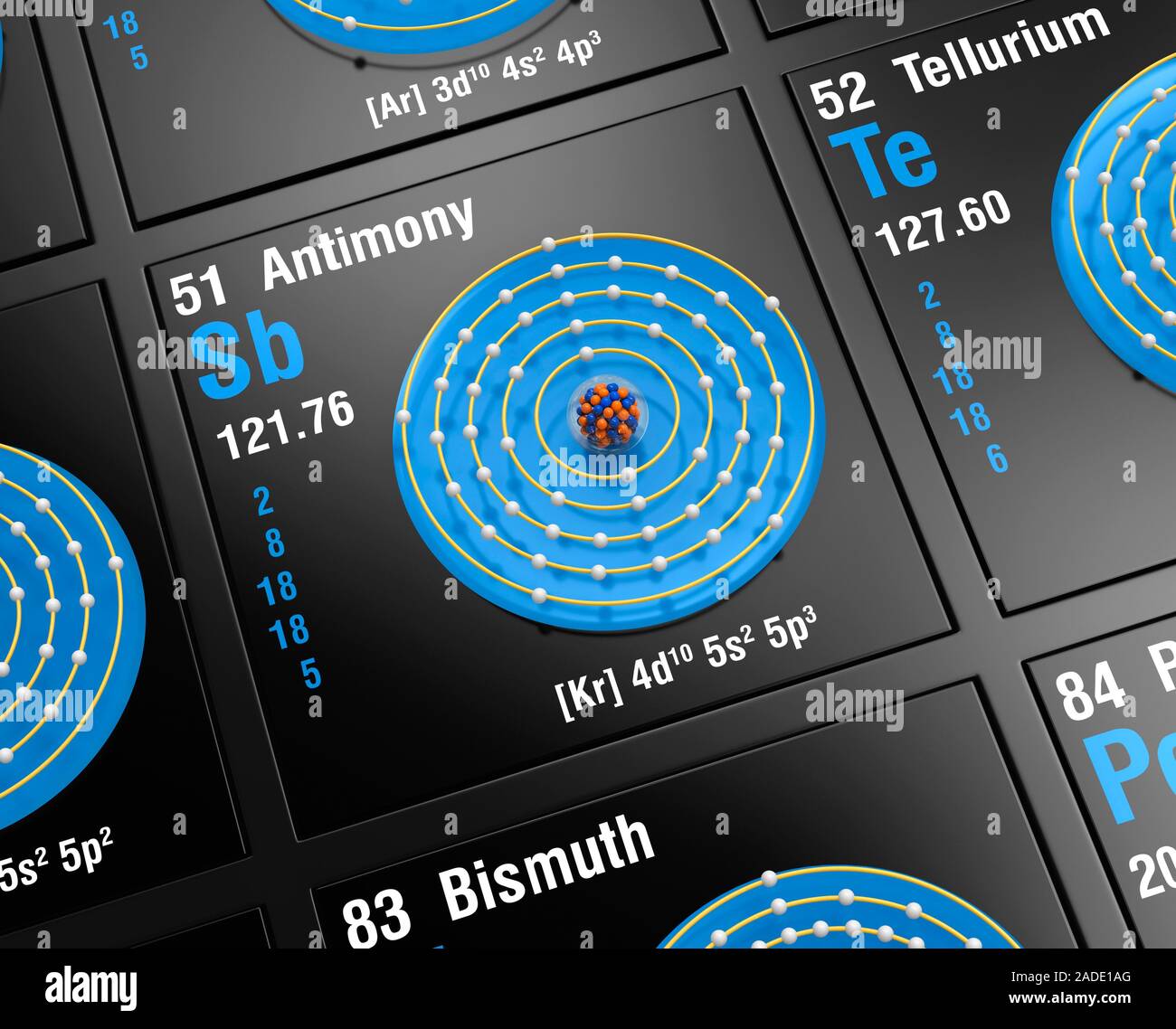
Diagram of the nuclear composition, electron configuration, and valence orbitals of an atom of antimony-122 (atomic number: 51), an isotope of this el
RMID:Image ID:2ADE1AG
Image details
Contributor:
Science Photo Library / Alamy Stock PhotoImage ID:
2ADE1AGFile size:
53.2 MB (1.2 MB Compressed download)Releases:
Model - no | Property - noDo I need a release?Dimensions:
4800 x 3872 px | 40.6 x 32.8 cm | 16 x 12.9 inches | 300dpiDate taken:
20 February 2019Photographer:
CARLOS CLARIVAN/SCIENCE PHOTO LIBRARYMore information:
Diagram of the nuclear composition, electron configuration, and valence orbitals of an atom of antimony-122 (atomic number: 51), an isotope of this element. The nucleus consists of 51 protons (blue) and 71 neutrons (red). The stability of an element's outer (valence) electrons determines its chemical and physical properties. Antimony is a metalloid in group 15, period 5, and the p-block of the periodic table. This toxic metal has a melting point of 630 degrees Celsius. It is used in alloys, with its compounds used in fire retardants.