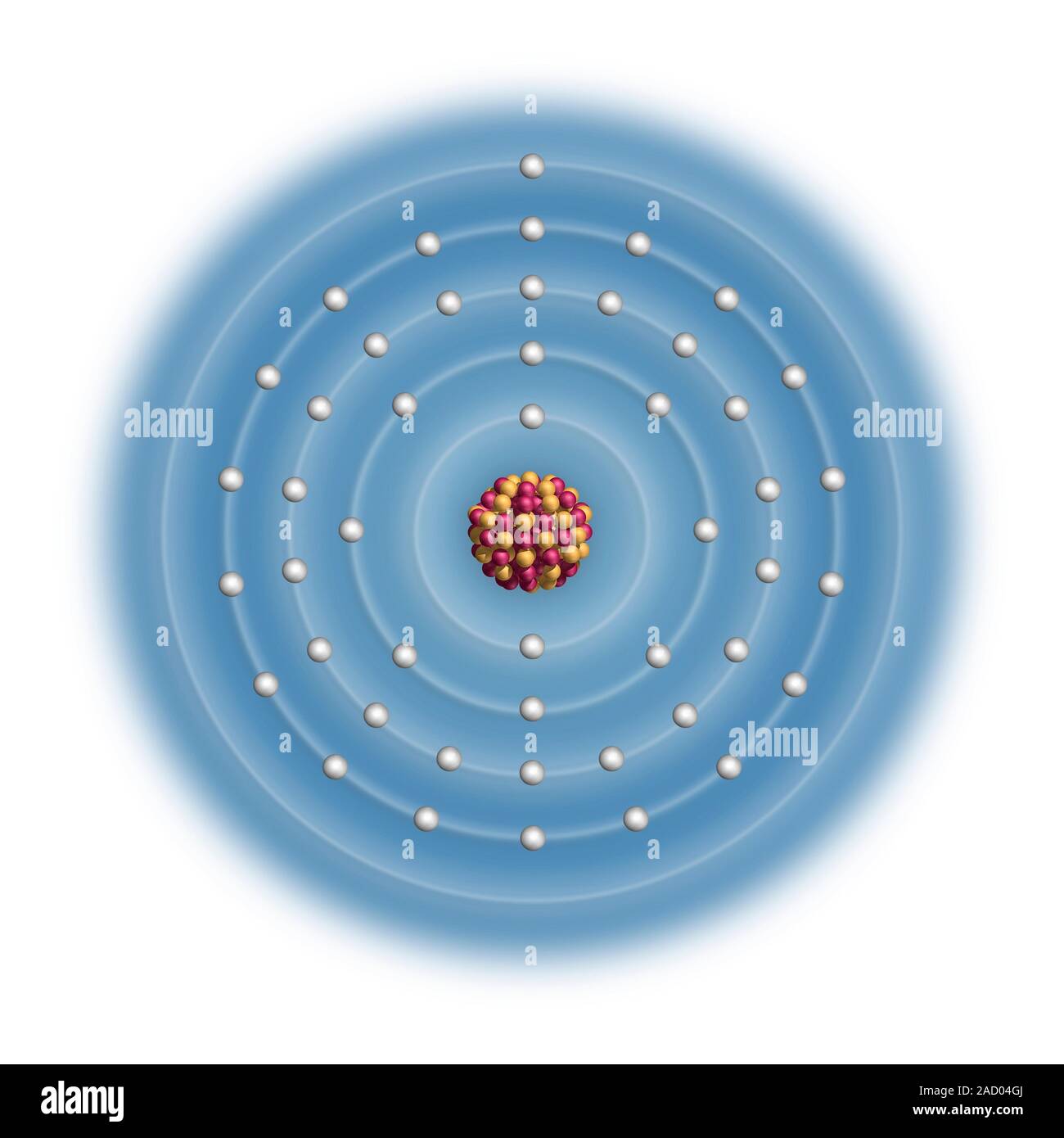
Silver (Ag). Diagram of the nuclear composition and electron configuration of an atom of silver-108 (atomic number: 47), a rare radioisotope of this e
RMID:Image ID:2AD04GJ
Image details
Contributor:
Science Photo Library / Alamy Stock PhotoImage ID:
2AD04GJFile size:
50 MB (428.9 KB Compressed download)Releases:
Model - no | Property - noDo I need a release?Dimensions:
4180 x 4180 px | 35.4 x 35.4 cm | 13.9 x 13.9 inches | 300dpiDate taken:
13 January 2015Photographer:
CARLOS CLARIVAN/SCIENCE PHOTO LIBRARYMore information:
Silver (Ag). Diagram of the nuclear composition and electron configuration of an atom of silver-108 (atomic number: 47), a rare radioisotope of this element. The nucleus consists of 47 protons (red) and 61 neutrons (yellow). 47 electrons (white) bind to the nucleus, successively occupying available electron shells (rings). Silver is a transition metal in group 11, period 5, and the d-block of the periodic table. It has a melting point of 962 degrees Celsius. The trends across the transition metals are due to electrons filling an inner d-subshell (here, within the 4th ring), shielding the outer electrons from the increasing nuclear charge.