Quick filters:
Ammonia sulphuric acid Stock Photos and Images
 bottles of salts acids and alkalis Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/stock-photo-bottles-of-salts-acids-and-alkalis-16464327.html
bottles of salts acids and alkalis Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/stock-photo-bottles-of-salts-acids-and-alkalis-16464327.htmlRMAW4KJG–bottles of salts acids and alkalis
 . Canadian machinery and metalworking (January-June 1919). // any advertisement interests you, tear it out now and place with letters to be answered. CANADIAN MACHINERY Volume XXI RAILS Open Hearth duality (All Sections from 121b to 100 lbs per yard) SPLICE, BARS STEEL TIE PLATES PIG IRON BASICFOUNDRY-^ESSEMER SULPHATE OF AMMONIA. Sulphuric Acid. Nitre Cake. BLOOMSBILLETS,SLABS. STRUCTURAL STEEL MERCHANT BARS CONCRETEREINFORCING BARS IRON, BRASSAND BRONZE CASTINGS Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/canadian-machinery-and-metalworking-january-june-1919-any-advertisement-interests-you-tear-it-out-now-and-place-with-letters-to-be-answered-canadian-machinery-volume-xxi-rails-open-hearth-duality-all-sections-from-121b-to-100-lbs-per-yard-splice-bars-steel-tie-plates-pig-iron-basicfoundry-essemer-sulphate-of-ammonia-sulphuric-acid-nitre-cake-bloomsbilletsslabs-structural-steel-merchant-bars-concretereinforcing-bars-iron-brassand-bronze-castings-image336761593.html
. Canadian machinery and metalworking (January-June 1919). // any advertisement interests you, tear it out now and place with letters to be answered. CANADIAN MACHINERY Volume XXI RAILS Open Hearth duality (All Sections from 121b to 100 lbs per yard) SPLICE, BARS STEEL TIE PLATES PIG IRON BASICFOUNDRY-^ESSEMER SULPHATE OF AMMONIA. Sulphuric Acid. Nitre Cake. BLOOMSBILLETS,SLABS. STRUCTURAL STEEL MERCHANT BARS CONCRETEREINFORCING BARS IRON, BRASSAND BRONZE CASTINGS Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/canadian-machinery-and-metalworking-january-june-1919-any-advertisement-interests-you-tear-it-out-now-and-place-with-letters-to-be-answered-canadian-machinery-volume-xxi-rails-open-hearth-duality-all-sections-from-121b-to-100-lbs-per-yard-splice-bars-steel-tie-plates-pig-iron-basicfoundry-essemer-sulphate-of-ammonia-sulphuric-acid-nitre-cake-bloomsbilletsslabs-structural-steel-merchant-bars-concretereinforcing-bars-iron-brassand-bronze-castings-image336761593.htmlRM2AFTPRN–. Canadian machinery and metalworking (January-June 1919). // any advertisement interests you, tear it out now and place with letters to be answered. CANADIAN MACHINERY Volume XXI RAILS Open Hearth duality (All Sections from 121b to 100 lbs per yard) SPLICE, BARS STEEL TIE PLATES PIG IRON BASICFOUNDRY-^ESSEMER SULPHATE OF AMMONIA. Sulphuric Acid. Nitre Cake. BLOOMSBILLETS,SLABS. STRUCTURAL STEEL MERCHANT BARS CONCRETEREINFORCING BARS IRON, BRASSAND BRONZE CASTINGS
 By-products developed from coal by G. H. Davis Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/by-products-developed-from-coal-by-g-h-davis-image501432040.html
By-products developed from coal by G. H. Davis Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/by-products-developed-from-coal-by-g-h-davis-image501432040.htmlRM2M3P5K4–By-products developed from coal by G. H. Davis
 When once ignited it burns as readily as the vit reous variety. any substance soluble in water for which sulphuric acid (diluted) has a stronger affinity than for iron lead tin and zinc? A. Your questions are rather indefinite. All of the alkalies-soda potassa ammonia etc.-also some of the alkaline earths as lime baryta or strontia are more or less soluble in water and have stronger affinities for sulphuric acid than iron. 2. Also any substances ity than for copper? A. If we understand you most of the metallic sulphates are soluble in water and are not decomposed by strong oil of vitriol. If Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/when-once-ignited-it-burns-as-readily-as-the-vit-reous-variety-any-substance-soluble-in-water-for-which-sulphuric-acid-diluted-has-a-stronger-affinity-than-for-iron-lead-tin-and-zinc-a-your-questions-are-rather-indefinite-all-of-the-alkalies-soda-potassa-ammonia-etc-also-some-of-the-alkaline-earths-as-lime-baryta-or-strontia-are-more-or-less-soluble-in-water-and-have-stronger-affinities-for-sulphuric-acid-than-iron-2-also-any-substances-ity-than-for-copper-a-if-we-understand-you-most-of-the-metallic-sulphates-are-soluble-in-water-and-are-not-decomposed-by-strong-oil-of-vitriol-if-image334319663.html
When once ignited it burns as readily as the vit reous variety. any substance soluble in water for which sulphuric acid (diluted) has a stronger affinity than for iron lead tin and zinc? A. Your questions are rather indefinite. All of the alkalies-soda potassa ammonia etc.-also some of the alkaline earths as lime baryta or strontia are more or less soluble in water and have stronger affinities for sulphuric acid than iron. 2. Also any substances ity than for copper? A. If we understand you most of the metallic sulphates are soluble in water and are not decomposed by strong oil of vitriol. If Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/when-once-ignited-it-burns-as-readily-as-the-vit-reous-variety-any-substance-soluble-in-water-for-which-sulphuric-acid-diluted-has-a-stronger-affinity-than-for-iron-lead-tin-and-zinc-a-your-questions-are-rather-indefinite-all-of-the-alkalies-soda-potassa-ammonia-etc-also-some-of-the-alkaline-earths-as-lime-baryta-or-strontia-are-more-or-less-soluble-in-water-and-have-stronger-affinities-for-sulphuric-acid-than-iron-2-also-any-substances-ity-than-for-copper-a-if-we-understand-you-most-of-the-metallic-sulphates-are-soluble-in-water-and-are-not-decomposed-by-strong-oil-of-vitriol-if-image334319663.htmlRM2ABWG3Y–When once ignited it burns as readily as the vit reous variety. any substance soluble in water for which sulphuric acid (diluted) has a stronger affinity than for iron lead tin and zinc? A. Your questions are rather indefinite. All of the alkalies-soda potassa ammonia etc.-also some of the alkaline earths as lime baryta or strontia are more or less soluble in water and have stronger affinities for sulphuric acid than iron. 2. Also any substances ity than for copper? A. If we understand you most of the metallic sulphates are soluble in water and are not decomposed by strong oil of vitriol. If
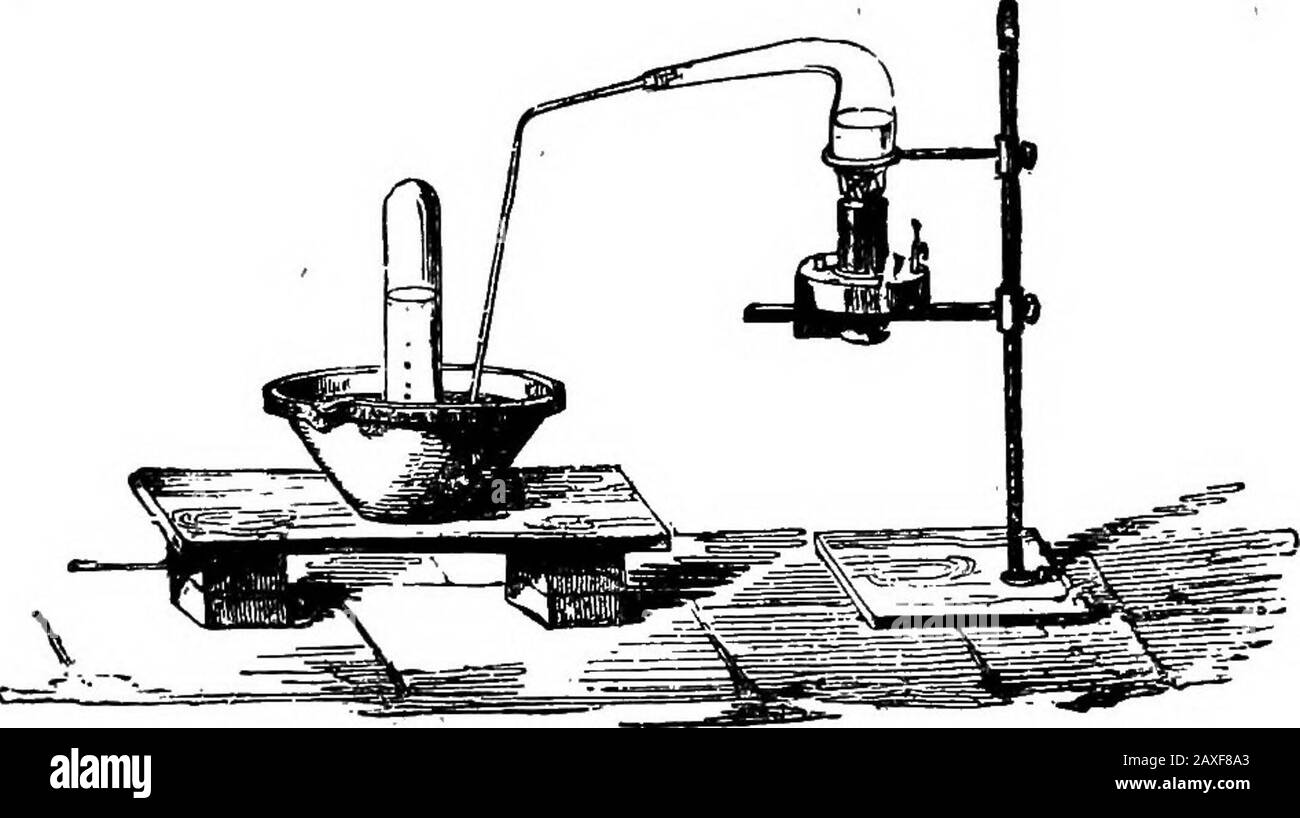 Scientific amusements . Experiment to obtain nitric aqid. from the nitrate of ammonia, which, on the application ofheftt, decomposes into nitrous oxide and vapour. Warm 86 CHEMISTRY. water should be used for the trough. The gas is apowerful supporter of combustion. Binoxide of nitrogen is of importance in the manufac-ture of sulphuric acid. Nitrogen combines with hydrogen, forming variouscompounds. These are the amines, also ammonia, andammonium. Ammonia possesses the properties of a base.Its name is derived from Jupiter Ammon, near whosetemple it was prepared, from camels dung. But bodiescont Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/scientific-amusements-experiment-to-obtain-nitric-aqid-from-the-nitrate-of-ammonia-which-on-the-application-ofheftt-decomposes-into-nitrous-oxide-and-vapour-warm-86-chemistry-water-should-be-used-for-the-trough-the-gas-is-apowerful-supporter-of-combustion-binoxide-of-nitrogen-is-of-importance-in-the-manufac-ture-of-sulphuric-acid-nitrogen-combines-with-hydrogen-forming-variouscompounds-these-are-the-amines-also-ammonia-andammonium-ammonia-possesses-the-properties-of-a-baseits-name-is-derived-from-jupiter-ammon-near-whosetemple-it-was-prepared-from-camels-dung-but-bodiescont-image343313883.html
Scientific amusements . Experiment to obtain nitric aqid. from the nitrate of ammonia, which, on the application ofheftt, decomposes into nitrous oxide and vapour. Warm 86 CHEMISTRY. water should be used for the trough. The gas is apowerful supporter of combustion. Binoxide of nitrogen is of importance in the manufac-ture of sulphuric acid. Nitrogen combines with hydrogen, forming variouscompounds. These are the amines, also ammonia, andammonium. Ammonia possesses the properties of a base.Its name is derived from Jupiter Ammon, near whosetemple it was prepared, from camels dung. But bodiescont Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/scientific-amusements-experiment-to-obtain-nitric-aqid-from-the-nitrate-of-ammonia-which-on-the-application-ofheftt-decomposes-into-nitrous-oxide-and-vapour-warm-86-chemistry-water-should-be-used-for-the-trough-the-gas-is-apowerful-supporter-of-combustion-binoxide-of-nitrogen-is-of-importance-in-the-manufac-ture-of-sulphuric-acid-nitrogen-combines-with-hydrogen-forming-variouscompounds-these-are-the-amines-also-ammonia-andammonium-ammonia-possesses-the-properties-of-a-baseits-name-is-derived-from-jupiter-ammon-near-whosetemple-it-was-prepared-from-camels-dung-but-bodiescont-image343313883.htmlRM2AXF8A3–Scientific amusements . Experiment to obtain nitric aqid. from the nitrate of ammonia, which, on the application ofheftt, decomposes into nitrous oxide and vapour. Warm 86 CHEMISTRY. water should be used for the trough. The gas is apowerful supporter of combustion. Binoxide of nitrogen is of importance in the manufac-ture of sulphuric acid. Nitrogen combines with hydrogen, forming variouscompounds. These are the amines, also ammonia, andammonium. Ammonia possesses the properties of a base.Its name is derived from Jupiter Ammon, near whosetemple it was prepared, from camels dung. But bodiescont
 Scientific amusements . Cavendishs experiment. with heart disease should not use it without advice, how-ever. When inhaled into the lungs it makes the subjectvery hilarious, and the effect is rather nojsy, It is obtained. Experiment to obtain nitric aqid. from the nitrate of ammonia, which, on the application ofheftt, decomposes into nitrous oxide and vapour. Warm 86 CHEMISTRY. water should be used for the trough. The gas is apowerful supporter of combustion. Binoxide of nitrogen is of importance in the manufac-ture of sulphuric acid. Nitrogen combines with hydrogen, forming variouscompounds. Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/scientific-amusements-cavendishs-experiment-with-heart-disease-should-not-use-it-without-advice-how-ever-when-inhaled-into-the-lungs-it-makes-the-subjectvery-hilarious-and-the-effect-is-rather-nojsy-it-is-obtained-experiment-to-obtain-nitric-aqid-from-the-nitrate-of-ammonia-which-on-the-application-ofheftt-decomposes-into-nitrous-oxide-and-vapour-warm-86-chemistry-water-should-be-used-for-the-trough-the-gas-is-apowerful-supporter-of-combustion-binoxide-of-nitrogen-is-of-importance-in-the-manufac-ture-of-sulphuric-acid-nitrogen-combines-with-hydrogen-forming-variouscompounds-image343313966.html
Scientific amusements . Cavendishs experiment. with heart disease should not use it without advice, how-ever. When inhaled into the lungs it makes the subjectvery hilarious, and the effect is rather nojsy, It is obtained. Experiment to obtain nitric aqid. from the nitrate of ammonia, which, on the application ofheftt, decomposes into nitrous oxide and vapour. Warm 86 CHEMISTRY. water should be used for the trough. The gas is apowerful supporter of combustion. Binoxide of nitrogen is of importance in the manufac-ture of sulphuric acid. Nitrogen combines with hydrogen, forming variouscompounds. Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/scientific-amusements-cavendishs-experiment-with-heart-disease-should-not-use-it-without-advice-how-ever-when-inhaled-into-the-lungs-it-makes-the-subjectvery-hilarious-and-the-effect-is-rather-nojsy-it-is-obtained-experiment-to-obtain-nitric-aqid-from-the-nitrate-of-ammonia-which-on-the-application-ofheftt-decomposes-into-nitrous-oxide-and-vapour-warm-86-chemistry-water-should-be-used-for-the-trough-the-gas-is-apowerful-supporter-of-combustion-binoxide-of-nitrogen-is-of-importance-in-the-manufac-ture-of-sulphuric-acid-nitrogen-combines-with-hydrogen-forming-variouscompounds-image343313966.htmlRM2AXF8D2–Scientific amusements . Cavendishs experiment. with heart disease should not use it without advice, how-ever. When inhaled into the lungs it makes the subjectvery hilarious, and the effect is rather nojsy, It is obtained. Experiment to obtain nitric aqid. from the nitrate of ammonia, which, on the application ofheftt, decomposes into nitrous oxide and vapour. Warm 86 CHEMISTRY. water should be used for the trough. The gas is apowerful supporter of combustion. Binoxide of nitrogen is of importance in the manufac-ture of sulphuric acid. Nitrogen combines with hydrogen, forming variouscompounds.
 . Mechanical appliances, mechanical movements and novelties of construction; a complete work and a continuation, as a second volume, of the author's book entitled 'Mechanical movements, powers and devices' ... including an explanatory chapter on the leading conceptions of perpetual motion existing during the past three centuries. cid liquor, circu-lated by pumps, containing sulphate of ammonia with about 4 per centexcess of free sulphuric acid. Combination of the ammonia of the gas with the free acid takesplace, giving still more sulphate of ammonia, so that to make theprocess continuous, some Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/mechanical-appliances-mechanical-movements-and-novelties-of-construction-a-complete-work-and-a-continuation-as-a-second-volume-of-the-authors-book-entitled-mechanical-movements-powers-and-devices-including-an-explanatory-chapter-on-the-leading-conceptions-of-perpetual-motion-existing-during-the-past-three-centuries-cid-liquor-circu-lated-by-pumps-containing-sulphate-of-ammonia-with-about-4-per-centexcess-of-free-sulphuric-acid-combination-of-the-ammonia-of-the-gas-with-the-free-acid-takesplace-giving-still-more-sulphate-of-ammonia-so-that-to-make-theprocess-continuous-some-image336963701.html
. Mechanical appliances, mechanical movements and novelties of construction; a complete work and a continuation, as a second volume, of the author's book entitled 'Mechanical movements, powers and devices' ... including an explanatory chapter on the leading conceptions of perpetual motion existing during the past three centuries. cid liquor, circu-lated by pumps, containing sulphate of ammonia with about 4 per centexcess of free sulphuric acid. Combination of the ammonia of the gas with the free acid takesplace, giving still more sulphate of ammonia, so that to make theprocess continuous, some Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/mechanical-appliances-mechanical-movements-and-novelties-of-construction-a-complete-work-and-a-continuation-as-a-second-volume-of-the-authors-book-entitled-mechanical-movements-powers-and-devices-including-an-explanatory-chapter-on-the-leading-conceptions-of-perpetual-motion-existing-during-the-past-three-centuries-cid-liquor-circu-lated-by-pumps-containing-sulphate-of-ammonia-with-about-4-per-centexcess-of-free-sulphuric-acid-combination-of-the-ammonia-of-the-gas-with-the-free-acid-takesplace-giving-still-more-sulphate-of-ammonia-so-that-to-make-theprocess-continuous-some-image336963701.htmlRM2AG60HW–. Mechanical appliances, mechanical movements and novelties of construction; a complete work and a continuation, as a second volume, of the author's book entitled 'Mechanical movements, powers and devices' ... including an explanatory chapter on the leading conceptions of perpetual motion existing during the past three centuries. cid liquor, circu-lated by pumps, containing sulphate of ammonia with about 4 per centexcess of free sulphuric acid. Combination of the ammonia of the gas with the free acid takesplace, giving still more sulphate of ammonia, so that to make theprocess continuous, some
 Knight's American mechanical dictionary : a description of tools, instruments, machines, processes and engineering, history of inventions, general technological vocabulary ; and digest of mechanical appliances in science and the arts . the operation by artifi-cial draft ; the facilitating of the operation by thelessening of the atmo.spheric pressure ; tlie alisorp-tion of the evolved vapor by sulphuric acid. Wehave now to consider the use of materials more vol-atile than water, notably ether and ammonia, butcommon alcohol, ether, methylic ether or alcohol,sulpburous or carbonic acid, bisulphid Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/knights-american-mechanical-dictionary-a-description-of-tools-instruments-machines-processes-and-engineering-history-of-inventions-general-technological-vocabulary-and-digest-of-mechanical-appliances-in-science-and-the-arts-the-operation-by-artifi-cial-draft-the-facilitating-of-the-operation-by-thelessening-of-the-atmospheric-pressure-tlie-alisorp-tion-of-the-evolved-vapor-by-sulphuric-acid-wehave-now-to-consider-the-use-of-materials-more-vol-atile-than-water-notably-ether-and-ammonia-butcommon-alcohol-ether-methylic-ether-or-alcoholsulpburous-or-carbonic-acid-bisulphid-image340007202.html
Knight's American mechanical dictionary : a description of tools, instruments, machines, processes and engineering, history of inventions, general technological vocabulary ; and digest of mechanical appliances in science and the arts . the operation by artifi-cial draft ; the facilitating of the operation by thelessening of the atmo.spheric pressure ; tlie alisorp-tion of the evolved vapor by sulphuric acid. Wehave now to consider the use of materials more vol-atile than water, notably ether and ammonia, butcommon alcohol, ether, methylic ether or alcohol,sulpburous or carbonic acid, bisulphid Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/knights-american-mechanical-dictionary-a-description-of-tools-instruments-machines-processes-and-engineering-history-of-inventions-general-technological-vocabulary-and-digest-of-mechanical-appliances-in-science-and-the-arts-the-operation-by-artifi-cial-draft-the-facilitating-of-the-operation-by-thelessening-of-the-atmospheric-pressure-tlie-alisorp-tion-of-the-evolved-vapor-by-sulphuric-acid-wehave-now-to-consider-the-use-of-materials-more-vol-atile-than-water-notably-ether-and-ammonia-butcommon-alcohol-ether-methylic-ether-or-alcoholsulpburous-or-carbonic-acid-bisulphid-image340007202.htmlRM2AN4JJA–Knight's American mechanical dictionary : a description of tools, instruments, machines, processes and engineering, history of inventions, general technological vocabulary ; and digest of mechanical appliances in science and the arts . the operation by artifi-cial draft ; the facilitating of the operation by thelessening of the atmo.spheric pressure ; tlie alisorp-tion of the evolved vapor by sulphuric acid. Wehave now to consider the use of materials more vol-atile than water, notably ether and ammonia, butcommon alcohol, ether, methylic ether or alcohol,sulpburous or carbonic acid, bisulphid
 A supplement to Ures Dictionary of Arts, Manufactures, and Mines, : containing a clear exposition of their principles and practice. . ,it will dissolve in sulphuric acid, and the addition of sulphate of potash or ammonia willconvert it into potash or ammonia-alum. Its formula, according Jo Graham, is a basic alum, HO SO+ 3(A10S0)-f OHO.By losing alumina it becomes the neutral salt. Sulphate of Alumina.—The first step towards the production of alum is the sulphate ofalumina. This is found in various proportions in alum stone. The pure mineral has thefollowing composition:— 1 atom of alumina - - Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/a-supplement-to-ures-dictionary-of-arts-manufactures-and-mines-containing-a-clear-exposition-of-their-principles-and-practice-it-will-dissolve-in-sulphuric-acid-and-the-addition-of-sulphate-of-potash-or-ammonia-willconvert-it-into-potash-or-ammonia-alum-its-formula-according-jo-graham-is-a-basic-alum-ho-so-3a10s0-f-ohoby-losing-alumina-it-becomes-the-neutral-salt-sulphate-of-aluminathe-first-step-towards-the-production-of-alum-is-the-sulphate-ofalumina-this-is-found-in-various-proportions-in-alum-stone-the-pure-mineral-has-thefollowing-composition-1-atom-of-alumina-image340019717.html
A supplement to Ures Dictionary of Arts, Manufactures, and Mines, : containing a clear exposition of their principles and practice. . ,it will dissolve in sulphuric acid, and the addition of sulphate of potash or ammonia willconvert it into potash or ammonia-alum. Its formula, according Jo Graham, is a basic alum, HO SO+ 3(A10S0)-f OHO.By losing alumina it becomes the neutral salt. Sulphate of Alumina.—The first step towards the production of alum is the sulphate ofalumina. This is found in various proportions in alum stone. The pure mineral has thefollowing composition:— 1 atom of alumina - - Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/a-supplement-to-ures-dictionary-of-arts-manufactures-and-mines-containing-a-clear-exposition-of-their-principles-and-practice-it-will-dissolve-in-sulphuric-acid-and-the-addition-of-sulphate-of-potash-or-ammonia-willconvert-it-into-potash-or-ammonia-alum-its-formula-according-jo-graham-is-a-basic-alum-ho-so-3a10s0-f-ohoby-losing-alumina-it-becomes-the-neutral-salt-sulphate-of-aluminathe-first-step-towards-the-production-of-alum-is-the-sulphate-ofalumina-this-is-found-in-various-proportions-in-alum-stone-the-pure-mineral-has-thefollowing-composition-1-atom-of-alumina-image340019717.htmlRM2AN56H9–A supplement to Ures Dictionary of Arts, Manufactures, and Mines, : containing a clear exposition of their principles and practice. . ,it will dissolve in sulphuric acid, and the addition of sulphate of potash or ammonia willconvert it into potash or ammonia-alum. Its formula, according Jo Graham, is a basic alum, HO SO+ 3(A10S0)-f OHO.By losing alumina it becomes the neutral salt. Sulphate of Alumina.—The first step towards the production of alum is the sulphate ofalumina. This is found in various proportions in alum stone. The pure mineral has thefollowing composition:— 1 atom of alumina - -
 Diagnostic methods, chemical, bacteriological and microscopical, a text-book for students and practitioners . inthe vessel C. At the end of this time the bell-jar is removed, the acid titratedwith tenth-normal sodium hydrate, and the number of c.c. of remaining aciddetermined. One c.c. of tenth-normal sulphuric acid neutralized by theevolved ammonia represents 0.001704 gram of ammonia. This figure is 230 DIAGNOSTIC METHODS. multiplied by 4 to obtain the percentage ammonia value. If any moisture ispresent on the inside of the bell-jar it should be washed into the sulphuricacid before titration. Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/diagnostic-methods-chemical-bacteriological-and-microscopical-a-text-book-for-students-and-practitioners-inthe-vessel-c-at-the-end-of-this-time-the-bell-jar-is-removed-the-acid-titratedwith-tenth-normal-sodium-hydrate-and-the-number-of-cc-of-remaining-aciddetermined-one-cc-of-tenth-normal-sulphuric-acid-neutralized-by-theevolved-ammonia-represents-0001704-gram-of-ammonia-this-figure-is-230-diagnostic-methods-multiplied-by-4-to-obtain-the-percentage-ammonia-value-if-any-moisture-ispresent-on-the-inside-of-the-bell-jar-it-should-be-washed-into-the-sulphuricacid-before-titration-image339286570.html
Diagnostic methods, chemical, bacteriological and microscopical, a text-book for students and practitioners . inthe vessel C. At the end of this time the bell-jar is removed, the acid titratedwith tenth-normal sodium hydrate, and the number of c.c. of remaining aciddetermined. One c.c. of tenth-normal sulphuric acid neutralized by theevolved ammonia represents 0.001704 gram of ammonia. This figure is 230 DIAGNOSTIC METHODS. multiplied by 4 to obtain the percentage ammonia value. If any moisture ispresent on the inside of the bell-jar it should be washed into the sulphuricacid before titration. Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/diagnostic-methods-chemical-bacteriological-and-microscopical-a-text-book-for-students-and-practitioners-inthe-vessel-c-at-the-end-of-this-time-the-bell-jar-is-removed-the-acid-titratedwith-tenth-normal-sodium-hydrate-and-the-number-of-cc-of-remaining-aciddetermined-one-cc-of-tenth-normal-sulphuric-acid-neutralized-by-theevolved-ammonia-represents-0001704-gram-of-ammonia-this-figure-is-230-diagnostic-methods-multiplied-by-4-to-obtain-the-percentage-ammonia-value-if-any-moisture-ispresent-on-the-inside-of-the-bell-jar-it-should-be-washed-into-the-sulphuricacid-before-titration-image339286570.htmlRM2AKYRDE–Diagnostic methods, chemical, bacteriological and microscopical, a text-book for students and practitioners . inthe vessel C. At the end of this time the bell-jar is removed, the acid titratedwith tenth-normal sodium hydrate, and the number of c.c. of remaining aciddetermined. One c.c. of tenth-normal sulphuric acid neutralized by theevolved ammonia represents 0.001704 gram of ammonia. This figure is 230 DIAGNOSTIC METHODS. multiplied by 4 to obtain the percentage ammonia value. If any moisture ispresent on the inside of the bell-jar it should be washed into the sulphuricacid before titration.
 A supplement to Ures Dictionary of Arts, Manufactures, and Mines, : containing a clear exposition of their principles and practice. . n be mixed with potash, Picolincis detected by its peculinr odor. Naphthaline is discovered not only by its odor, but mayalso be separated Ijy sublimation or heating, after converting the ammonia in the solutioninto a salt by sulphuric or hydrochloric acid.—Dr. Maclogan. We imported into England of sulphate and liquor of ammonia as follows:— Ammonia, sulphate of.Ammonia, liquor. 1856,1855,1855, lbs. 23,904 343,609 22,400 Since, for the purpose of purification on Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/a-supplement-to-ures-dictionary-of-arts-manufactures-and-mines-containing-a-clear-exposition-of-their-principles-and-practice-n-be-mixed-with-potash-picolincis-detected-by-its-peculinr-odor-naphthaline-is-discovered-not-only-by-its-odor-but-mayalso-be-separated-ijy-sublimation-or-heating-after-converting-the-ammonia-in-the-solutioninto-a-salt-by-sulphuric-or-hydrochloric-aciddr-maclogan-we-imported-into-england-of-sulphate-and-liquor-of-ammonia-as-follows-ammonia-sulphate-ofammonia-liquor-185618551855-lbs-23904-343609-22400-since-for-the-purpose-of-purification-on-image340019416.html
A supplement to Ures Dictionary of Arts, Manufactures, and Mines, : containing a clear exposition of their principles and practice. . n be mixed with potash, Picolincis detected by its peculinr odor. Naphthaline is discovered not only by its odor, but mayalso be separated Ijy sublimation or heating, after converting the ammonia in the solutioninto a salt by sulphuric or hydrochloric acid.—Dr. Maclogan. We imported into England of sulphate and liquor of ammonia as follows:— Ammonia, sulphate of.Ammonia, liquor. 1856,1855,1855, lbs. 23,904 343,609 22,400 Since, for the purpose of purification on Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/a-supplement-to-ures-dictionary-of-arts-manufactures-and-mines-containing-a-clear-exposition-of-their-principles-and-practice-n-be-mixed-with-potash-picolincis-detected-by-its-peculinr-odor-naphthaline-is-discovered-not-only-by-its-odor-but-mayalso-be-separated-ijy-sublimation-or-heating-after-converting-the-ammonia-in-the-solutioninto-a-salt-by-sulphuric-or-hydrochloric-aciddr-maclogan-we-imported-into-england-of-sulphate-and-liquor-of-ammonia-as-follows-ammonia-sulphate-ofammonia-liquor-185618551855-lbs-23904-343609-22400-since-for-the-purpose-of-purification-on-image340019416.htmlRM2AN566G–A supplement to Ures Dictionary of Arts, Manufactures, and Mines, : containing a clear exposition of their principles and practice. . n be mixed with potash, Picolincis detected by its peculinr odor. Naphthaline is discovered not only by its odor, but mayalso be separated Ijy sublimation or heating, after converting the ammonia in the solutioninto a salt by sulphuric or hydrochloric acid.—Dr. Maclogan. We imported into England of sulphate and liquor of ammonia as follows:— Ammonia, sulphate of.Ammonia, liquor. 1856,1855,1855, lbs. 23,904 343,609 22,400 Since, for the purpose of purification on
![A manual of photographic chemistry, theoretical and practical . development being complete, it is of little consequencewhether the resulting image be red, green, or blue. The tone?can be changed in the simplest manner. But before doing soit is advisable to fix the picture by washing off the solublesalts. This is done in common water. Immerse the print inwater acidulated with nitric or sulphuric acid; quickly theoriginal color, whatever it may have been, will change to a deepbluish green. Wash the print and again immerse it in watercontaining a few dro])s of ammonia, when, almost instantly, the Stock Photo A manual of photographic chemistry, theoretical and practical . development being complete, it is of little consequencewhether the resulting image be red, green, or blue. The tone?can be changed in the simplest manner. But before doing soit is advisable to fix the picture by washing off the solublesalts. This is done in common water. Immerse the print inwater acidulated with nitric or sulphuric acid; quickly theoriginal color, whatever it may have been, will change to a deepbluish green. Wash the print and again immerse it in watercontaining a few dro])s of ammonia, when, almost instantly, the Stock Photo](https://c8.alamy.com/comp/2ANFAT0/a-manual-of-photographic-chemistry-theoretical-and-practical-development-being-complete-it-is-of-little-consequencewhether-the-resulting-image-be-red-green-or-blue-the-tonecan-be-changed-in-the-simplest-manner-but-before-doing-soit-is-advisable-to-fix-the-picture-by-washing-off-the-solublesalts-this-is-done-in-common-water-immerse-the-print-inwater-acidulated-with-nitric-or-sulphuric-acid-quickly-theoriginal-color-whatever-it-may-have-been-will-change-to-a-deepbluish-green-wash-the-print-and-again-immerse-it-in-watercontaining-a-few-dro-s-of-ammonia-when-almost-instantly-the-2ANFAT0.jpg) A manual of photographic chemistry, theoretical and practical . development being complete, it is of little consequencewhether the resulting image be red, green, or blue. The tone?can be changed in the simplest manner. But before doing soit is advisable to fix the picture by washing off the solublesalts. This is done in common water. Immerse the print inwater acidulated with nitric or sulphuric acid; quickly theoriginal color, whatever it may have been, will change to a deepbluish green. Wash the print and again immerse it in watercontaining a few dro])s of ammonia, when, almost instantly, the Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/a-manual-of-photographic-chemistry-theoretical-and-practical-development-being-complete-it-is-of-little-consequencewhether-the-resulting-image-be-red-green-or-blue-the-tonecan-be-changed-in-the-simplest-manner-but-before-doing-soit-is-advisable-to-fix-the-picture-by-washing-off-the-solublesalts-this-is-done-in-common-water-immerse-the-print-inwater-acidulated-with-nitric-or-sulphuric-acid-quickly-theoriginal-color-whatever-it-may-have-been-will-change-to-a-deepbluish-green-wash-the-print-and-again-immerse-it-in-watercontaining-a-few-dro-s-of-ammonia-when-almost-instantly-the-image340242560.html
A manual of photographic chemistry, theoretical and practical . development being complete, it is of little consequencewhether the resulting image be red, green, or blue. The tone?can be changed in the simplest manner. But before doing soit is advisable to fix the picture by washing off the solublesalts. This is done in common water. Immerse the print inwater acidulated with nitric or sulphuric acid; quickly theoriginal color, whatever it may have been, will change to a deepbluish green. Wash the print and again immerse it in watercontaining a few dro])s of ammonia, when, almost instantly, the Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/a-manual-of-photographic-chemistry-theoretical-and-practical-development-being-complete-it-is-of-little-consequencewhether-the-resulting-image-be-red-green-or-blue-the-tonecan-be-changed-in-the-simplest-manner-but-before-doing-soit-is-advisable-to-fix-the-picture-by-washing-off-the-solublesalts-this-is-done-in-common-water-immerse-the-print-inwater-acidulated-with-nitric-or-sulphuric-acid-quickly-theoriginal-color-whatever-it-may-have-been-will-change-to-a-deepbluish-green-wash-the-print-and-again-immerse-it-in-watercontaining-a-few-dro-s-of-ammonia-when-almost-instantly-the-image340242560.htmlRM2ANFAT0–A manual of photographic chemistry, theoretical and practical . development being complete, it is of little consequencewhether the resulting image be red, green, or blue. The tone?can be changed in the simplest manner. But before doing soit is advisable to fix the picture by washing off the solublesalts. This is done in common water. Immerse the print inwater acidulated with nitric or sulphuric acid; quickly theoriginal color, whatever it may have been, will change to a deepbluish green. Wash the print and again immerse it in watercontaining a few dro])s of ammonia, when, almost instantly, the
 A manual of practical hygiene for students, physicians, and health officers . ater made slightly acid with sulphuric acid. The first 25-50 cc. ofdistillate shoukl be rejected, and the next 50 cc. should be tested withNesslers reagent. If no color appears, the distillate is ammonia-free,and the operation may then be continued until the contents of the re-tort are reduced to very small volume. If the test shows traces ofammonia, successive portions should be tested until a negative resultis secured. It is well to prepare a goodly supply, and to keep it onhand in glass-st<)pj)ered bi)ttles. Ap Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/a-manual-of-practical-hygiene-for-students-physicians-and-health-officers-ater-made-slightly-acid-with-sulphuric-acid-the-first-25-50-cc-ofdistillate-shoukl-be-rejected-and-the-next-50-cc-should-be-tested-withnesslers-reagent-if-no-color-appears-the-distillate-is-ammonia-freeand-the-operation-may-then-be-continued-until-the-contents-of-the-re-tort-are-reduced-to-very-small-volume-if-the-test-shows-traces-ofammonia-successive-portions-should-be-tested-until-a-negative-resultis-secured-it-is-well-to-prepare-a-goodly-supply-and-to-keep-it-onhand-in-glass-stltpjered-bittles-ap-image339013264.html
A manual of practical hygiene for students, physicians, and health officers . ater made slightly acid with sulphuric acid. The first 25-50 cc. ofdistillate shoukl be rejected, and the next 50 cc. should be tested withNesslers reagent. If no color appears, the distillate is ammonia-free,and the operation may then be continued until the contents of the re-tort are reduced to very small volume. If the test shows traces ofammonia, successive portions should be tested until a negative resultis secured. It is well to prepare a goodly supply, and to keep it onhand in glass-st<)pj)ered bi)ttles. Ap Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/a-manual-of-practical-hygiene-for-students-physicians-and-health-officers-ater-made-slightly-acid-with-sulphuric-acid-the-first-25-50-cc-ofdistillate-shoukl-be-rejected-and-the-next-50-cc-should-be-tested-withnesslers-reagent-if-no-color-appears-the-distillate-is-ammonia-freeand-the-operation-may-then-be-continued-until-the-contents-of-the-re-tort-are-reduced-to-very-small-volume-if-the-test-shows-traces-ofammonia-successive-portions-should-be-tested-until-a-negative-resultis-secured-it-is-well-to-prepare-a-goodly-supply-and-to-keep-it-onhand-in-glass-stltpjered-bittles-ap-image339013264.htmlRM2AKFATG–A manual of practical hygiene for students, physicians, and health officers . ater made slightly acid with sulphuric acid. The first 25-50 cc. ofdistillate shoukl be rejected, and the next 50 cc. should be tested withNesslers reagent. If no color appears, the distillate is ammonia-free,and the operation may then be continued until the contents of the re-tort are reduced to very small volume. If the test shows traces ofammonia, successive portions should be tested until a negative resultis secured. It is well to prepare a goodly supply, and to keep it onhand in glass-st<)pj)ered bi)ttles. Ap
 . Chemistry: general, medical, and pharmaceutical, including the chemistry of the U. S. Pharmacopia. A manual on the general principles of the science, and their applications in medicine and pharmacy. oride. 310 Calcium. Ammonium oxalate. 117 Chlorides. Silver nitrate. 268 Ammonia. Nesslers reagent. 639 Aqua Destillata, Carbonic acid. Lime-water. 314 Organic matter. Potassium permanganate. 652 Nitrates. Diphenylamine and sul-phuric acid (ring). Nitrites. Zinc, iodide, starch (blue). Aqua Hydrogenii fDioxidi, 1 Hydrofluoric acid. Sulphuric acid. 342 Barium. Dilute sulphuric acid. 103 Copper.Lea Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/chemistry-general-medical-and-pharmaceutical-including-the-chemistry-of-the-u-s-pharmacopia-a-manual-on-the-general-principles-of-the-science-and-their-applications-in-medicine-and-pharmacy-oride-310-calcium-ammonium-oxalate-117-chlorides-silver-nitrate-268-ammonia-nesslers-reagent-639-aqua-destillata-carbonic-acid-lime-water-314-organic-matter-potassium-permanganate-652-nitrates-diphenylamine-and-sul-phuric-acid-ring-nitrites-zinc-iodide-starch-blue-aqua-hydrogenii-fdioxidi-1-hydrofluoric-acid-sulphuric-acid-342-barium-dilute-sulphuric-acid-103-copperlea-image337053511.html
. Chemistry: general, medical, and pharmaceutical, including the chemistry of the U. S. Pharmacopia. A manual on the general principles of the science, and their applications in medicine and pharmacy. oride. 310 Calcium. Ammonium oxalate. 117 Chlorides. Silver nitrate. 268 Ammonia. Nesslers reagent. 639 Aqua Destillata, Carbonic acid. Lime-water. 314 Organic matter. Potassium permanganate. 652 Nitrates. Diphenylamine and sul-phuric acid (ring). Nitrites. Zinc, iodide, starch (blue). Aqua Hydrogenii fDioxidi, 1 Hydrofluoric acid. Sulphuric acid. 342 Barium. Dilute sulphuric acid. 103 Copper.Lea Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/chemistry-general-medical-and-pharmaceutical-including-the-chemistry-of-the-u-s-pharmacopia-a-manual-on-the-general-principles-of-the-science-and-their-applications-in-medicine-and-pharmacy-oride-310-calcium-ammonium-oxalate-117-chlorides-silver-nitrate-268-ammonia-nesslers-reagent-639-aqua-destillata-carbonic-acid-lime-water-314-organic-matter-potassium-permanganate-652-nitrates-diphenylamine-and-sul-phuric-acid-ring-nitrites-zinc-iodide-starch-blue-aqua-hydrogenii-fdioxidi-1-hydrofluoric-acid-sulphuric-acid-342-barium-dilute-sulphuric-acid-103-copperlea-image337053511.htmlRM2AGA35B–. Chemistry: general, medical, and pharmaceutical, including the chemistry of the U. S. Pharmacopia. A manual on the general principles of the science, and their applications in medicine and pharmacy. oride. 310 Calcium. Ammonium oxalate. 117 Chlorides. Silver nitrate. 268 Ammonia. Nesslers reagent. 639 Aqua Destillata, Carbonic acid. Lime-water. 314 Organic matter. Potassium permanganate. 652 Nitrates. Diphenylamine and sul-phuric acid (ring). Nitrites. Zinc, iodide, starch (blue). Aqua Hydrogenii fDioxidi, 1 Hydrofluoric acid. Sulphuric acid. 342 Barium. Dilute sulphuric acid. 103 Copper.Lea
 The Gardeners' Chronicle and Agricultural Gazette . e, Fenchmch-street. /:jENUINE PERUVIAN GUANO, the ImportationVJ of Messrs. A. GIBBS & SONS, London ; or Mr. MYERS,Liverpool. Strongest Sulphuric Acid, Sulphate of Soda forPotatoes, Sulphate and Phosphate of Ammonia, Superphosphateof Lime, Gj^jsum, Salt, and every other Artificial Manure.—London Manure Company, 40, New Bridge-street. E. Purser,Secretary. mo FARMERS, MARKET-GARDENERS. FLOR--L ISTS, and persons in the habit of using GUANO in aliquid state.—An opportunity which seldom occms ; the Adver-tizer having a large quantity of the above,a Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/the-gardeners-chronicle-and-agricultural-gazette-e-fenchmch-street-jenuine-peruvian-guano-the-importationvj-of-messrs-a-gibbs-sons-london-or-mr-myersliverpool-strongest-sulphuric-acid-sulphate-of-soda-forpotatoes-sulphate-and-phosphate-of-ammonia-superphosphateof-lime-gjjsum-salt-and-every-other-artificial-manurelondon-manure-company-40-new-bridge-street-e-pursersecretary-mo-farmers-market-gardeners-flor-l-ists-and-persons-in-the-habit-of-using-guano-in-aliquid-statean-opportunity-which-seldom-occms-the-adver-tizer-having-a-large-quantity-of-the-abovea-image338262264.html
The Gardeners' Chronicle and Agricultural Gazette . e, Fenchmch-street. /:jENUINE PERUVIAN GUANO, the ImportationVJ of Messrs. A. GIBBS & SONS, London ; or Mr. MYERS,Liverpool. Strongest Sulphuric Acid, Sulphate of Soda forPotatoes, Sulphate and Phosphate of Ammonia, Superphosphateof Lime, Gj^jsum, Salt, and every other Artificial Manure.—London Manure Company, 40, New Bridge-street. E. Purser,Secretary. mo FARMERS, MARKET-GARDENERS. FLOR--L ISTS, and persons in the habit of using GUANO in aliquid state.—An opportunity which seldom occms ; the Adver-tizer having a large quantity of the above,a Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/the-gardeners-chronicle-and-agricultural-gazette-e-fenchmch-street-jenuine-peruvian-guano-the-importationvj-of-messrs-a-gibbs-sons-london-or-mr-myersliverpool-strongest-sulphuric-acid-sulphate-of-soda-forpotatoes-sulphate-and-phosphate-of-ammonia-superphosphateof-lime-gjjsum-salt-and-every-other-artificial-manurelondon-manure-company-40-new-bridge-street-e-pursersecretary-mo-farmers-market-gardeners-flor-l-ists-and-persons-in-the-habit-of-using-guano-in-aliquid-statean-opportunity-which-seldom-occms-the-adver-tizer-having-a-large-quantity-of-the-abovea-image338262264.htmlRM2AJ94Y4–The Gardeners' Chronicle and Agricultural Gazette . e, Fenchmch-street. /:jENUINE PERUVIAN GUANO, the ImportationVJ of Messrs. A. GIBBS & SONS, London ; or Mr. MYERS,Liverpool. Strongest Sulphuric Acid, Sulphate of Soda forPotatoes, Sulphate and Phosphate of Ammonia, Superphosphateof Lime, Gj^jsum, Salt, and every other Artificial Manure.—London Manure Company, 40, New Bridge-street. E. Purser,Secretary. mo FARMERS, MARKET-GARDENERS. FLOR--L ISTS, and persons in the habit of using GUANO in aliquid state.—An opportunity which seldom occms ; the Adver-tizer having a large quantity of the above,a
 Chemical lecture experiments . ssed through a glass AMMONIA 191 tube thrust through a three-holed cork in the neck of a smallbottle containing a 2 cm. layer of sulphuric acid. A streamof nitric oxide (Ex. 49, p. 211) passes through a second glasstube into the bottle. Both tubes dip beneath the surface ofthe sulphuric acid that their rate of bubbling may be noticed.Hydrogen is conducted throughthe whole apparatus to drive outall air and then the nitric oxidegenerator started. The current ofhydrogen should be three timesas fast as the current of nitricoxide. The mixed gases are thenconducted thr Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/chemical-lecture-experiments-ssed-through-a-glass-ammonia-191-tube-thrust-through-a-three-holed-cork-in-the-neck-of-a-smallbottle-containing-a-2-cm-layer-of-sulphuric-acid-a-streamof-nitric-oxide-ex-49-p-211-passes-through-a-second-glasstube-into-the-bottle-both-tubes-dip-beneath-the-surface-ofthe-sulphuric-acid-that-their-rate-of-bubbling-may-be-noticedhydrogen-is-conducted-throughthe-whole-apparatus-to-drive-outall-air-and-then-the-nitric-oxidegenerator-started-the-current-ofhydrogen-should-be-three-timesas-fast-as-the-current-of-nitricoxide-the-mixed-gases-are-thenconducted-thr-image343366030.html
Chemical lecture experiments . ssed through a glass AMMONIA 191 tube thrust through a three-holed cork in the neck of a smallbottle containing a 2 cm. layer of sulphuric acid. A streamof nitric oxide (Ex. 49, p. 211) passes through a second glasstube into the bottle. Both tubes dip beneath the surface ofthe sulphuric acid that their rate of bubbling may be noticed.Hydrogen is conducted throughthe whole apparatus to drive outall air and then the nitric oxidegenerator started. The current ofhydrogen should be three timesas fast as the current of nitricoxide. The mixed gases are thenconducted thr Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/chemical-lecture-experiments-ssed-through-a-glass-ammonia-191-tube-thrust-through-a-three-holed-cork-in-the-neck-of-a-smallbottle-containing-a-2-cm-layer-of-sulphuric-acid-a-streamof-nitric-oxide-ex-49-p-211-passes-through-a-second-glasstube-into-the-bottle-both-tubes-dip-beneath-the-surface-ofthe-sulphuric-acid-that-their-rate-of-bubbling-may-be-noticedhydrogen-is-conducted-throughthe-whole-apparatus-to-drive-outall-air-and-then-the-nitric-oxidegenerator-started-the-current-ofhydrogen-should-be-three-timesas-fast-as-the-current-of-nitricoxide-the-mixed-gases-are-thenconducted-thr-image343366030.htmlRM2AXHJTE–Chemical lecture experiments . ssed through a glass AMMONIA 191 tube thrust through a three-holed cork in the neck of a smallbottle containing a 2 cm. layer of sulphuric acid. A streamof nitric oxide (Ex. 49, p. 211) passes through a second glasstube into the bottle. Both tubes dip beneath the surface ofthe sulphuric acid that their rate of bubbling may be noticed.Hydrogen is conducted throughthe whole apparatus to drive outall air and then the nitric oxidegenerator started. The current ofhydrogen should be three timesas fast as the current of nitricoxide. The mixed gases are thenconducted thr
 Report of the Committee on Editing Tentative and Offical Methods of Analysis, the Association of Official Agricultural Chemists . r N/50 sulphuric or XXIl MEAT AND MEAT PRODUCTS 275 hydrochloric acid in tube (E). Then add 1 cc. of saturated potassium oxalate solu-tion to the sample in tube (B), introduce a few drops of kerosene and finally addjust sufficient saturated potassium carbonate solution to render the mixture alkaline.Place the tubes in position at once, pass air through the apparatus and titrate thestandard acid in tube (E) at hourly intervals, until ammonia ceases to be given off,us Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/report-of-the-committee-on-editing-tentative-and-offical-methods-of-analysis-the-association-of-official-agricultural-chemists-r-n50-sulphuric-or-xxil-meat-and-meat-products-275-hydrochloric-acid-in-tube-e-then-add-1-cc-of-saturated-potassium-oxalate-solu-tion-to-the-sample-in-tube-b-introduce-a-few-drops-of-kerosene-and-finally-addjust-sufficient-saturated-potassium-carbonate-solution-to-render-the-mixture-alkalineplace-the-tubes-in-position-at-once-pass-air-through-the-apparatus-and-titrate-thestandard-acid-in-tube-e-at-hourly-intervals-until-ammonia-ceases-to-be-given-offus-image338146530.html
Report of the Committee on Editing Tentative and Offical Methods of Analysis, the Association of Official Agricultural Chemists . r N/50 sulphuric or XXIl MEAT AND MEAT PRODUCTS 275 hydrochloric acid in tube (E). Then add 1 cc. of saturated potassium oxalate solu-tion to the sample in tube (B), introduce a few drops of kerosene and finally addjust sufficient saturated potassium carbonate solution to render the mixture alkaline.Place the tubes in position at once, pass air through the apparatus and titrate thestandard acid in tube (E) at hourly intervals, until ammonia ceases to be given off,us Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/report-of-the-committee-on-editing-tentative-and-offical-methods-of-analysis-the-association-of-official-agricultural-chemists-r-n50-sulphuric-or-xxil-meat-and-meat-products-275-hydrochloric-acid-in-tube-e-then-add-1-cc-of-saturated-potassium-oxalate-solu-tion-to-the-sample-in-tube-b-introduce-a-few-drops-of-kerosene-and-finally-addjust-sufficient-saturated-potassium-carbonate-solution-to-render-the-mixture-alkalineplace-the-tubes-in-position-at-once-pass-air-through-the-apparatus-and-titrate-thestandard-acid-in-tube-e-at-hourly-intervals-until-ammonia-ceases-to-be-given-offus-image338146530.htmlRM2AJ3W9P–Report of the Committee on Editing Tentative and Offical Methods of Analysis, the Association of Official Agricultural Chemists . r N/50 sulphuric or XXIl MEAT AND MEAT PRODUCTS 275 hydrochloric acid in tube (E). Then add 1 cc. of saturated potassium oxalate solu-tion to the sample in tube (B), introduce a few drops of kerosene and finally addjust sufficient saturated potassium carbonate solution to render the mixture alkaline.Place the tubes in position at once, pass air through the apparatus and titrate thestandard acid in tube (E) at hourly intervals, until ammonia ceases to be given off,us
 . Canadian machinery and metalworking (January-June 1919). Steel Billets,Track Spikes &Bolts, Forgi rigs, Wireof every description. If any advertisement interests you, tear it out now and place with letters to be answered. CANADIAN MACHINERY Volume XXI. hi* mm m&, mmm BLOOMSBILLETS,SLABS. STRUCTURAL STEEL MERCHANT BARS CONCRETEREINFORCING IRON, BRASSAND BRONZE mm**. CASTINGS Sulphuric Acid. Nitre Cake >TCEL RAILS Open Hearth duality (All Sect ions from 12 lb to 100lbs per yard) SPLICE, BARS STEEL, TIE PLATES PIG IRON BASIC, FOUNDRY-BESSEMER SULPHATEOF AMMONIA // what you need is not adverti Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/canadian-machinery-and-metalworking-january-june-1919-steel-billetstrack-spikes-bolts-forgi-rigs-wireof-every-description-if-any-advertisement-interests-you-tear-it-out-now-and-place-with-letters-to-be-answered-canadian-machinery-volume-xxi-hi-mm-m-mmm-bloomsbilletsslabs-structural-steel-merchant-bars-concretereinforcing-iron-brassand-bronze-mm-castings-sulphuric-acid-nitre-cake-gttcel-rails-open-hearth-duality-all-sect-ions-from-12-lb-to-100lbs-per-yard-splice-bars-steel-tie-plates-pig-iron-basic-foundry-bessemer-sulphateof-ammonia-what-you-need-is-not-adverti-image336911771.html
. Canadian machinery and metalworking (January-June 1919). Steel Billets,Track Spikes &Bolts, Forgi rigs, Wireof every description. If any advertisement interests you, tear it out now and place with letters to be answered. CANADIAN MACHINERY Volume XXI. hi* mm m&, mmm BLOOMSBILLETS,SLABS. STRUCTURAL STEEL MERCHANT BARS CONCRETEREINFORCING IRON, BRASSAND BRONZE mm**. CASTINGS Sulphuric Acid. Nitre Cake >TCEL RAILS Open Hearth duality (All Sect ions from 12 lb to 100lbs per yard) SPLICE, BARS STEEL, TIE PLATES PIG IRON BASIC, FOUNDRY-BESSEMER SULPHATEOF AMMONIA // what you need is not adverti Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/canadian-machinery-and-metalworking-january-june-1919-steel-billetstrack-spikes-bolts-forgi-rigs-wireof-every-description-if-any-advertisement-interests-you-tear-it-out-now-and-place-with-letters-to-be-answered-canadian-machinery-volume-xxi-hi-mm-m-mmm-bloomsbilletsslabs-structural-steel-merchant-bars-concretereinforcing-iron-brassand-bronze-mm-castings-sulphuric-acid-nitre-cake-gttcel-rails-open-hearth-duality-all-sect-ions-from-12-lb-to-100lbs-per-yard-splice-bars-steel-tie-plates-pig-iron-basic-foundry-bessemer-sulphateof-ammonia-what-you-need-is-not-adverti-image336911771.htmlRM2AG3JB7–. Canadian machinery and metalworking (January-June 1919). Steel Billets,Track Spikes &Bolts, Forgi rigs, Wireof every description. If any advertisement interests you, tear it out now and place with letters to be answered. CANADIAN MACHINERY Volume XXI. hi* mm m&, mmm BLOOMSBILLETS,SLABS. STRUCTURAL STEEL MERCHANT BARS CONCRETEREINFORCING IRON, BRASSAND BRONZE mm**. CASTINGS Sulphuric Acid. Nitre Cake >TCEL RAILS Open Hearth duality (All Sect ions from 12 lb to 100lbs per yard) SPLICE, BARS STEEL, TIE PLATES PIG IRON BASIC, FOUNDRY-BESSEMER SULPHATEOF AMMONIA // what you need is not adverti
 Wehman Bros.' new book of one hundred and fifty parlor tricks and games : home-made apparatus . d Red Liquid from the Same Bottle. Boil some leaves of red cabbage, and after half an hoursebullition you will have a beautiful purple liquid, whichwhen cold may be put in a bottle for future use. Takethree glasses. Let one be perfectly clean, in the second puta drop of ammonia, and in the third a drop of sulphuric acid.The liquid poured into the clean glass will, of course, pre-serve its original color, that in the second will turn green,and that in the third will become red. To Make a Needle Float Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/wehman-bros-new-book-of-one-hundred-and-fifty-parlor-tricks-and-games-home-made-apparatus-d-red-liquid-from-the-same-bottle-boil-some-leaves-of-red-cabbage-and-after-half-an-hoursebullition-you-will-have-a-beautiful-purple-liquid-whichwhen-cold-may-be-put-in-a-bottle-for-future-use-takethree-glasses-let-one-be-perfectly-clean-in-the-second-puta-drop-of-ammonia-and-in-the-third-a-drop-of-sulphuric-acidthe-liquid-poured-into-the-clean-glass-will-of-course-pre-serve-its-original-color-that-in-the-second-will-turn-greenand-that-in-the-third-will-become-red-to-make-a-needle-float-image342810043.html
Wehman Bros.' new book of one hundred and fifty parlor tricks and games : home-made apparatus . d Red Liquid from the Same Bottle. Boil some leaves of red cabbage, and after half an hoursebullition you will have a beautiful purple liquid, whichwhen cold may be put in a bottle for future use. Takethree glasses. Let one be perfectly clean, in the second puta drop of ammonia, and in the third a drop of sulphuric acid.The liquid poured into the clean glass will, of course, pre-serve its original color, that in the second will turn green,and that in the third will become red. To Make a Needle Float Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/wehman-bros-new-book-of-one-hundred-and-fifty-parlor-tricks-and-games-home-made-apparatus-d-red-liquid-from-the-same-bottle-boil-some-leaves-of-red-cabbage-and-after-half-an-hoursebullition-you-will-have-a-beautiful-purple-liquid-whichwhen-cold-may-be-put-in-a-bottle-for-future-use-takethree-glasses-let-one-be-perfectly-clean-in-the-second-puta-drop-of-ammonia-and-in-the-third-a-drop-of-sulphuric-acidthe-liquid-poured-into-the-clean-glass-will-of-course-pre-serve-its-original-color-that-in-the-second-will-turn-greenand-that-in-the-third-will-become-red-to-make-a-needle-float-image342810043.htmlRM2AWM9KR–Wehman Bros.' new book of one hundred and fifty parlor tricks and games : home-made apparatus . d Red Liquid from the Same Bottle. Boil some leaves of red cabbage, and after half an hoursebullition you will have a beautiful purple liquid, whichwhen cold may be put in a bottle for future use. Takethree glasses. Let one be perfectly clean, in the second puta drop of ammonia, and in the third a drop of sulphuric acid.The liquid poured into the clean glass will, of course, pre-serve its original color, that in the second will turn green,and that in the third will become red. To Make a Needle Float
 . Canadian machinery and metalworking (January-June 1919). CASTINGS Sulphuric Acid. Nitre Cake RAILS Open Hearth Quality (All Sections from 12Ik to 100 lbs per yard) SPLICE, BARS STEEL, TIE PLATES PIG IRON BASIC.FOUNDRY-BESSEMER SULPHATE OF AMMONIA January 23, 1919 C A N A I) I A N MACI1I X E K Y. Crucible AND Open Hearth Steel Tool SteelADrn brand cTcri AIUjU highspeed o 1LLL The John Illingworth Steel Co. 1856 Frankford,New York Office Phila.217 Broadway RALPH B. NORTON, AGENTMontreal, Canada Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/canadian-machinery-and-metalworking-january-june-1919-castings-sulphuric-acid-nitre-cake-rails-open-hearth-quality-all-sections-from-12ik-to-100-lbs-per-yard-splice-bars-steel-tie-plates-pig-iron-basicfoundry-bessemer-sulphate-of-ammonia-january-23-1919-c-a-n-a-i-i-a-n-maci1i-x-e-k-y-crucible-and-open-hearth-steel-tool-steeladrn-brand-ctcri-aiuju-highspeed-o-1lll-the-john-illingworth-steel-co-1856-frankfordnew-york-office-phila217-broadway-ralph-b-norton-agentmontreal-canada-image336979354.html
. Canadian machinery and metalworking (January-June 1919). CASTINGS Sulphuric Acid. Nitre Cake RAILS Open Hearth Quality (All Sections from 12Ik to 100 lbs per yard) SPLICE, BARS STEEL, TIE PLATES PIG IRON BASIC.FOUNDRY-BESSEMER SULPHATE OF AMMONIA January 23, 1919 C A N A I) I A N MACI1I X E K Y. Crucible AND Open Hearth Steel Tool SteelADrn brand cTcri AIUjU highspeed o 1LLL The John Illingworth Steel Co. 1856 Frankford,New York Office Phila.217 Broadway RALPH B. NORTON, AGENTMontreal, Canada Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/canadian-machinery-and-metalworking-january-june-1919-castings-sulphuric-acid-nitre-cake-rails-open-hearth-quality-all-sections-from-12ik-to-100-lbs-per-yard-splice-bars-steel-tie-plates-pig-iron-basicfoundry-bessemer-sulphate-of-ammonia-january-23-1919-c-a-n-a-i-i-a-n-maci1i-x-e-k-y-crucible-and-open-hearth-steel-tool-steeladrn-brand-ctcri-aiuju-highspeed-o-1lll-the-john-illingworth-steel-co-1856-frankfordnew-york-office-phila217-broadway-ralph-b-norton-agentmontreal-canada-image336979354.htmlRM2AG6MGX–. Canadian machinery and metalworking (January-June 1919). CASTINGS Sulphuric Acid. Nitre Cake RAILS Open Hearth Quality (All Sections from 12Ik to 100 lbs per yard) SPLICE, BARS STEEL, TIE PLATES PIG IRON BASIC.FOUNDRY-BESSEMER SULPHATE OF AMMONIA January 23, 1919 C A N A I) I A N MACI1I X E K Y. Crucible AND Open Hearth Steel Tool SteelADrn brand cTcri AIUjU highspeed o 1LLL The John Illingworth Steel Co. 1856 Frankford,New York Office Phila.217 Broadway RALPH B. NORTON, AGENTMontreal, Canada
 Scientific and applied pharmacognosy intended for the use of students in pharmacy, as a hand book for pharmacists, and as a reference book for food and drug analysts and pharmacologists . owed tostand, with frequent agitation, from four to twenty-four hours.The solution is then filtered into a small separatory funnel and 5 c.c.of a dilute sulphuric acid (0.5 per cent) added, and after separationof the aqueous solution the latter is diluted with 5 c.c. of water. 1 Ether, 60 c.c; alcohol, 7.5 c.c; chloroform, 30 c.c; ammonia, 2.5 c.c. Itshould be borne in mind in this connection that probably th Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/scientific-and-applied-pharmacognosy-intended-for-the-use-of-students-in-pharmacy-as-a-hand-book-for-pharmacists-and-as-a-reference-book-for-food-and-drug-analysts-and-pharmacologists-owed-tostand-with-frequent-agitation-from-four-to-twenty-four-hoursthe-solution-is-then-filtered-into-a-small-separatory-funnel-and-5-ccof-a-dilute-sulphuric-acid-05-per-cent-added-and-after-separationof-the-aqueous-solution-the-latter-is-diluted-with-5-cc-of-water-1-ether-60-cc-alcohol-75-cc-chloroform-30-cc-ammonia-25-cc-itshould-be-borne-in-mind-in-this-connection-that-probably-th-image338194845.html
Scientific and applied pharmacognosy intended for the use of students in pharmacy, as a hand book for pharmacists, and as a reference book for food and drug analysts and pharmacologists . owed tostand, with frequent agitation, from four to twenty-four hours.The solution is then filtered into a small separatory funnel and 5 c.c.of a dilute sulphuric acid (0.5 per cent) added, and after separationof the aqueous solution the latter is diluted with 5 c.c. of water. 1 Ether, 60 c.c; alcohol, 7.5 c.c; chloroform, 30 c.c; ammonia, 2.5 c.c. Itshould be borne in mind in this connection that probably th Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/scientific-and-applied-pharmacognosy-intended-for-the-use-of-students-in-pharmacy-as-a-hand-book-for-pharmacists-and-as-a-reference-book-for-food-and-drug-analysts-and-pharmacologists-owed-tostand-with-frequent-agitation-from-four-to-twenty-four-hoursthe-solution-is-then-filtered-into-a-small-separatory-funnel-and-5-ccof-a-dilute-sulphuric-acid-05-per-cent-added-and-after-separationof-the-aqueous-solution-the-latter-is-diluted-with-5-cc-of-water-1-ether-60-cc-alcohol-75-cc-chloroform-30-cc-ammonia-25-cc-itshould-be-borne-in-mind-in-this-connection-that-probably-th-image338194845.htmlRM2AJ62Y9–Scientific and applied pharmacognosy intended for the use of students in pharmacy, as a hand book for pharmacists, and as a reference book for food and drug analysts and pharmacologists . owed tostand, with frequent agitation, from four to twenty-four hours.The solution is then filtered into a small separatory funnel and 5 c.c.of a dilute sulphuric acid (0.5 per cent) added, and after separationof the aqueous solution the latter is diluted with 5 c.c. of water. 1 Ether, 60 c.c; alcohol, 7.5 c.c; chloroform, 30 c.c; ammonia, 2.5 c.c. Itshould be borne in mind in this connection that probably th
 . Canadian machinery and metalworking (January-June 1919). CASTINGS Sulphuric Acid. Nitre Cake >TCEL RAILS Open Hearth duality (All Sect ions from 12 lb to 100lbs per yard) SPLICE, BARS STEEL, TIE PLATES PIG IRON BASIC, FOUNDRY-BESSEMER SULPHATEOF AMMONIA // what you need is not advertised, consult our Buyers Directory and write advertisers listed under proper heading. January 30, 1919 CANADIAN M A C II I N E R Y. |ii HIGH SPEEDAND CARBONTWIST DRILLS IN these days tools greatly define the extent of yourproduction. The skilled mechanic, as good as hemay be, must be supplied with good tools t Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/canadian-machinery-and-metalworking-january-june-1919-castings-sulphuric-acid-nitre-cake-gttcel-rails-open-hearth-duality-all-sect-ions-from-12-lb-to-100lbs-per-yard-splice-bars-steel-tie-plates-pig-iron-basic-foundry-bessemer-sulphateof-ammonia-what-you-need-is-not-advertised-consult-our-buyers-directory-and-write-advertisers-listed-under-proper-heading-january-30-1919-canadian-m-a-c-ii-i-n-e-r-y-ii-high-speedand-carbontwist-drills-in-these-days-tools-greatly-define-the-extent-of-yourproduction-the-skilled-mechanic-as-good-as-hemay-be-must-be-supplied-with-good-tools-t-image336911329.html
. Canadian machinery and metalworking (January-June 1919). CASTINGS Sulphuric Acid. Nitre Cake >TCEL RAILS Open Hearth duality (All Sect ions from 12 lb to 100lbs per yard) SPLICE, BARS STEEL, TIE PLATES PIG IRON BASIC, FOUNDRY-BESSEMER SULPHATEOF AMMONIA // what you need is not advertised, consult our Buyers Directory and write advertisers listed under proper heading. January 30, 1919 CANADIAN M A C II I N E R Y. |ii HIGH SPEEDAND CARBONTWIST DRILLS IN these days tools greatly define the extent of yourproduction. The skilled mechanic, as good as hemay be, must be supplied with good tools t Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/canadian-machinery-and-metalworking-january-june-1919-castings-sulphuric-acid-nitre-cake-gttcel-rails-open-hearth-duality-all-sect-ions-from-12-lb-to-100lbs-per-yard-splice-bars-steel-tie-plates-pig-iron-basic-foundry-bessemer-sulphateof-ammonia-what-you-need-is-not-advertised-consult-our-buyers-directory-and-write-advertisers-listed-under-proper-heading-january-30-1919-canadian-m-a-c-ii-i-n-e-r-y-ii-high-speedand-carbontwist-drills-in-these-days-tools-greatly-define-the-extent-of-yourproduction-the-skilled-mechanic-as-good-as-hemay-be-must-be-supplied-with-good-tools-t-image336911329.htmlRM2AG3HRD–. Canadian machinery and metalworking (January-June 1919). CASTINGS Sulphuric Acid. Nitre Cake >TCEL RAILS Open Hearth duality (All Sect ions from 12 lb to 100lbs per yard) SPLICE, BARS STEEL, TIE PLATES PIG IRON BASIC, FOUNDRY-BESSEMER SULPHATEOF AMMONIA // what you need is not advertised, consult our Buyers Directory and write advertisers listed under proper heading. January 30, 1919 CANADIAN M A C II I N E R Y. |ii HIGH SPEEDAND CARBONTWIST DRILLS IN these days tools greatly define the extent of yourproduction. The skilled mechanic, as good as hemay be, must be supplied with good tools t
 . Canadian machinery and metalworking (January-June 1919). $13 26 Sheets, 3% lbs. sq. ft. .. 13 25 IS 26Sheets, 4 to 6 lbs. sq. ft. 12 50 12 60Cut sheets, %c per lb. extra.Cut sheets to size, lc per lb. extra. PLATING CHEMICALS. Acid, boracic Acid, hydrochloric Acid, nitric Acid, sulphuric Ammonia, aqua Ammonium carbonate Ammonium, chloride Ammonium hydrosulphuret .... Ammonium sulphate Arsenic, white Copper, carbonate, annhy Copper, sulphate Cobalt, sulphate Iron perchloride Lead acetate Nickel ammonium sulphate .... Nickel carbonate Nickel sulphate Potassium carbonate Potassium sulphide (sub Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/canadian-machinery-and-metalworking-january-june-1919-13-26-sheets-3-lbs-sq-ft-13-25-is-26sheets-4-to-6-lbs-sq-ft-12-50-12-60cut-sheets-c-per-lb-extracut-sheets-to-size-lc-per-lb-extra-plating-chemicals-acid-boracic-acid-hydrochloric-acid-nitric-acid-sulphuric-ammonia-aqua-ammonium-carbonate-ammonium-chloride-ammonium-hydrosulphuret-ammonium-sulphate-arsenic-white-copper-carbonate-annhy-copper-sulphate-cobalt-sulphate-iron-perchloride-lead-acetate-nickel-ammonium-sulphate-nickel-carbonate-nickel-sulphate-potassium-carbonate-potassium-sulphide-sub-image336733557.html
. Canadian machinery and metalworking (January-June 1919). $13 26 Sheets, 3% lbs. sq. ft. .. 13 25 IS 26Sheets, 4 to 6 lbs. sq. ft. 12 50 12 60Cut sheets, %c per lb. extra.Cut sheets to size, lc per lb. extra. PLATING CHEMICALS. Acid, boracic Acid, hydrochloric Acid, nitric Acid, sulphuric Ammonia, aqua Ammonium carbonate Ammonium, chloride Ammonium hydrosulphuret .... Ammonium sulphate Arsenic, white Copper, carbonate, annhy Copper, sulphate Cobalt, sulphate Iron perchloride Lead acetate Nickel ammonium sulphate .... Nickel carbonate Nickel sulphate Potassium carbonate Potassium sulphide (sub Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/canadian-machinery-and-metalworking-january-june-1919-13-26-sheets-3-lbs-sq-ft-13-25-is-26sheets-4-to-6-lbs-sq-ft-12-50-12-60cut-sheets-c-per-lb-extracut-sheets-to-size-lc-per-lb-extra-plating-chemicals-acid-boracic-acid-hydrochloric-acid-nitric-acid-sulphuric-ammonia-aqua-ammonium-carbonate-ammonium-chloride-ammonium-hydrosulphuret-ammonium-sulphate-arsenic-white-copper-carbonate-annhy-copper-sulphate-cobalt-sulphate-iron-perchloride-lead-acetate-nickel-ammonium-sulphate-nickel-carbonate-nickel-sulphate-potassium-carbonate-potassium-sulphide-sub-image336733557.htmlRM2AFRF2D–. Canadian machinery and metalworking (January-June 1919). $13 26 Sheets, 3% lbs. sq. ft. .. 13 25 IS 26Sheets, 4 to 6 lbs. sq. ft. 12 50 12 60Cut sheets, %c per lb. extra.Cut sheets to size, lc per lb. extra. PLATING CHEMICALS. Acid, boracic Acid, hydrochloric Acid, nitric Acid, sulphuric Ammonia, aqua Ammonium carbonate Ammonium, chloride Ammonium hydrosulphuret .... Ammonium sulphate Arsenic, white Copper, carbonate, annhy Copper, sulphate Cobalt, sulphate Iron perchloride Lead acetate Nickel ammonium sulphate .... Nickel carbonate Nickel sulphate Potassium carbonate Potassium sulphide (sub
 A glossary of mineralogy . 1-73. Fig. 280. Comp. Snjphate of ammonia, NH*, S + 2H=sulphuric acid 52-33, ammonia 34*67,water 12-00 = 100, Readily soluble in water. Attracts mois-ture from the atmosphere, and is entirelyvolatile at a high temperature. Localities. In fissu.res in the lavas ofEtna and Vesuvius, and the Lipari Isles.The lagunes near Sienna in Tuscany. Name. After Professor Mascagui, bywhom it was discovered. Masculine. See Gem. Masonite, C. T. Jackson. A variety ofChloritoid. Colour black. Lustre pearly.H. 6. S.G, 3-52, Analysis, by Whitney; ilica , . 28-27 dumina . 32-16 rotoxide Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/a-glossary-of-mineralogy-1-73-fig-280-comp-snjphate-of-ammonia-nh-s-2h=sulphuric-acid-52-33-ammonia-3467water-12-00-=-100-readily-soluble-in-water-attracts-mois-ture-from-the-atmosphere-and-is-entirelyvolatile-at-a-high-temperature-localities-in-fissures-in-the-lavas-ofetna-and-vesuvius-and-the-lipari-islesthe-lagunes-near-sienna-in-tuscany-name-after-professor-mascagui-bywhom-it-was-discovered-masculine-see-gem-masonite-c-t-jackson-a-variety-ofchloritoid-colour-black-lustre-pearlyh-6-sg-3-52-analysis-by-whitney-ilica-28-27-dumina-32-16-rotoxide-image338123581.html
A glossary of mineralogy . 1-73. Fig. 280. Comp. Snjphate of ammonia, NH*, S + 2H=sulphuric acid 52-33, ammonia 34*67,water 12-00 = 100, Readily soluble in water. Attracts mois-ture from the atmosphere, and is entirelyvolatile at a high temperature. Localities. In fissu.res in the lavas ofEtna and Vesuvius, and the Lipari Isles.The lagunes near Sienna in Tuscany. Name. After Professor Mascagui, bywhom it was discovered. Masculine. See Gem. Masonite, C. T. Jackson. A variety ofChloritoid. Colour black. Lustre pearly.H. 6. S.G, 3-52, Analysis, by Whitney; ilica , . 28-27 dumina . 32-16 rotoxide Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/a-glossary-of-mineralogy-1-73-fig-280-comp-snjphate-of-ammonia-nh-s-2h=sulphuric-acid-52-33-ammonia-3467water-12-00-=-100-readily-soluble-in-water-attracts-mois-ture-from-the-atmosphere-and-is-entirelyvolatile-at-a-high-temperature-localities-in-fissures-in-the-lavas-ofetna-and-vesuvius-and-the-lipari-islesthe-lagunes-near-sienna-in-tuscany-name-after-professor-mascagui-bywhom-it-was-discovered-masculine-see-gem-masonite-c-t-jackson-a-variety-ofchloritoid-colour-black-lustre-pearlyh-6-sg-3-52-analysis-by-whitney-ilica-28-27-dumina-32-16-rotoxide-image338123581.htmlRM2AJ2T25–A glossary of mineralogy . 1-73. Fig. 280. Comp. Snjphate of ammonia, NH*, S + 2H=sulphuric acid 52-33, ammonia 34*67,water 12-00 = 100, Readily soluble in water. Attracts mois-ture from the atmosphere, and is entirelyvolatile at a high temperature. Localities. In fissu.res in the lavas ofEtna and Vesuvius, and the Lipari Isles.The lagunes near Sienna in Tuscany. Name. After Professor Mascagui, bywhom it was discovered. Masculine. See Gem. Masonite, C. T. Jackson. A variety ofChloritoid. Colour black. Lustre pearly.H. 6. S.G, 3-52, Analysis, by Whitney; ilica , . 28-27 dumina . 32-16 rotoxide
 The housekeeper's companion : a practical receipt book and household physician, with much other valuable information . 01 Acid, sulphuric 402 Aconite 396 Alkalies 405 Ammonia 405 Animal poisons 396 Antimony 403 Aqua fortis 402 Arsenic. 403 Balladonna 397 Blue vitriol , 404 Butter of antimony 403 Calomel 404 Cambogia 398 Carbolic acid 402 Chloride of zinc 405 Copper 404 Corrosive sublimate 404 I>eadly nightshade 397 Digitalis 397 |Fox-glove 397 jOamboge 398 JEiellebores, the 398 Hemlock 398 Hemp, Indian 398 Henbane 399 Hydrochloric acid 402 Hyoscyamus 399 Indian hemp ^ 399 404 Mineral poison Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/the-housekeepers-companion-a-practical-receipt-book-and-household-physician-with-much-other-valuable-information-01-acid-sulphuric-402-aconite-396-alkalies-405-ammonia-405-animal-poisons-396-antimony-403-aqua-fortis-402-arsenic-403-balladonna-397-blue-vitriol-404-butter-of-antimony-403-calomel-404-cambogia-398-carbolic-acid-402-chloride-of-zinc-405-copper-404-corrosive-sublimate-404-igteadly-nightshade-397-digitalis-397-fox-glove-397-joamboge-398-jeiellebores-the-398-hemlock-398-hemp-indian-398-henbane-399-hydrochloric-acid-402-hyoscyamus-399-indian-hemp-399-404-mineral-poison-image339282308.html
The housekeeper's companion : a practical receipt book and household physician, with much other valuable information . 01 Acid, sulphuric 402 Aconite 396 Alkalies 405 Ammonia 405 Animal poisons 396 Antimony 403 Aqua fortis 402 Arsenic. 403 Balladonna 397 Blue vitriol , 404 Butter of antimony 403 Calomel 404 Cambogia 398 Carbolic acid 402 Chloride of zinc 405 Copper 404 Corrosive sublimate 404 I>eadly nightshade 397 Digitalis 397 |Fox-glove 397 jOamboge 398 JEiellebores, the 398 Hemlock 398 Hemp, Indian 398 Henbane 399 Hydrochloric acid 402 Hyoscyamus 399 Indian hemp ^ 399 404 Mineral poison Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/the-housekeepers-companion-a-practical-receipt-book-and-household-physician-with-much-other-valuable-information-01-acid-sulphuric-402-aconite-396-alkalies-405-ammonia-405-animal-poisons-396-antimony-403-aqua-fortis-402-arsenic-403-balladonna-397-blue-vitriol-404-butter-of-antimony-403-calomel-404-cambogia-398-carbolic-acid-402-chloride-of-zinc-405-copper-404-corrosive-sublimate-404-igteadly-nightshade-397-digitalis-397-fox-glove-397-joamboge-398-jeiellebores-the-398-hemlock-398-hemp-indian-398-henbane-399-hydrochloric-acid-402-hyoscyamus-399-indian-hemp-399-404-mineral-poison-image339282308.htmlRM2AKYJ18–The housekeeper's companion : a practical receipt book and household physician, with much other valuable information . 01 Acid, sulphuric 402 Aconite 396 Alkalies 405 Ammonia 405 Animal poisons 396 Antimony 403 Aqua fortis 402 Arsenic. 403 Balladonna 397 Blue vitriol , 404 Butter of antimony 403 Calomel 404 Cambogia 398 Carbolic acid 402 Chloride of zinc 405 Copper 404 Corrosive sublimate 404 I>eadly nightshade 397 Digitalis 397 |Fox-glove 397 jOamboge 398 JEiellebores, the 398 Hemlock 398 Hemp, Indian 398 Henbane 399 Hydrochloric acid 402 Hyoscyamus 399 Indian hemp ^ 399 404 Mineral poison
 . Canadian machinery and metalworking (January-June 1919). 3% lbs. sq. ft. .. 13 25 13 USheets, 4 to 6 lbs. sq. ft. 12 50 12 SOCut sheets, %c per lb. extra.Cut sheets to size, lc per lb. extra. PLATING CHEMICALS. Acid, boracic $ .25 Acid, hydrochloric 06 Acid, nitric 14 Acid, sulphuric 06 Ammonia, aqua 23 Ammonium carbonate Ammonium, chloride 55 Ammonium hydrosulphuret 30 Ammonium sulphate 15 Arsenic, white 27 Copper, carbonate, annhy 50 Copper, sulphate 22 Cobalt, sulphate 20 Iron perchloride 40 Lead acetate 35 Nickel ammonium sulphate .... -25 Nickel carbonate 32 Nickel sulphate 35 Potassium Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/canadian-machinery-and-metalworking-january-june-1919-3-lbs-sq-ft-13-25-13-usheets-4-to-6-lbs-sq-ft-12-50-12-socut-sheets-c-per-lb-extracut-sheets-to-size-lc-per-lb-extra-plating-chemicals-acid-boracic-25-acid-hydrochloric-06-acid-nitric-14-acid-sulphuric-06-ammonia-aqua-23-ammonium-carbonate-ammonium-chloride-55-ammonium-hydrosulphuret-30-ammonium-sulphate-15-arsenic-white-27-copper-carbonate-annhy-50-copper-sulphate-22-cobalt-sulphate-20-iron-perchloride-40-lead-acetate-35-nickel-ammonium-sulphate-25-nickel-carbonate-32-nickel-sulphate-35-potassium-image336834796.html
. Canadian machinery and metalworking (January-June 1919). 3% lbs. sq. ft. .. 13 25 13 USheets, 4 to 6 lbs. sq. ft. 12 50 12 SOCut sheets, %c per lb. extra.Cut sheets to size, lc per lb. extra. PLATING CHEMICALS. Acid, boracic $ .25 Acid, hydrochloric 06 Acid, nitric 14 Acid, sulphuric 06 Ammonia, aqua 23 Ammonium carbonate Ammonium, chloride 55 Ammonium hydrosulphuret 30 Ammonium sulphate 15 Arsenic, white 27 Copper, carbonate, annhy 50 Copper, sulphate 22 Cobalt, sulphate 20 Iron perchloride 40 Lead acetate 35 Nickel ammonium sulphate .... -25 Nickel carbonate 32 Nickel sulphate 35 Potassium Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/canadian-machinery-and-metalworking-january-june-1919-3-lbs-sq-ft-13-25-13-usheets-4-to-6-lbs-sq-ft-12-50-12-socut-sheets-c-per-lb-extracut-sheets-to-size-lc-per-lb-extra-plating-chemicals-acid-boracic-25-acid-hydrochloric-06-acid-nitric-14-acid-sulphuric-06-ammonia-aqua-23-ammonium-carbonate-ammonium-chloride-55-ammonium-hydrosulphuret-30-ammonium-sulphate-15-arsenic-white-27-copper-carbonate-annhy-50-copper-sulphate-22-cobalt-sulphate-20-iron-perchloride-40-lead-acetate-35-nickel-ammonium-sulphate-25-nickel-carbonate-32-nickel-sulphate-35-potassium-image336834796.htmlRM2AG0464–. Canadian machinery and metalworking (January-June 1919). 3% lbs. sq. ft. .. 13 25 13 USheets, 4 to 6 lbs. sq. ft. 12 50 12 SOCut sheets, %c per lb. extra.Cut sheets to size, lc per lb. extra. PLATING CHEMICALS. Acid, boracic $ .25 Acid, hydrochloric 06 Acid, nitric 14 Acid, sulphuric 06 Ammonia, aqua 23 Ammonium carbonate Ammonium, chloride 55 Ammonium hydrosulphuret 30 Ammonium sulphate 15 Arsenic, white 27 Copper, carbonate, annhy 50 Copper, sulphate 22 Cobalt, sulphate 20 Iron perchloride 40 Lead acetate 35 Nickel ammonium sulphate .... -25 Nickel carbonate 32 Nickel sulphate 35 Potassium
 . Canadian machinery and metalworking (January-June 1919). onto Sheets, 3 lbs. sq. ft $13 25 $18 25 Sheets, 3% lbs. sq. ft. .. 13 25 18 26Sheets, 4 to 6 lbs. sq. ft. 12 50 12 60Cut sheets, %c per lb. extra.Cut sheets to size, lc per lb. extra. PLATING CHEMICALS. Acid, boracic $ 25 Acid, hydrochloric 06 Acid, nitric 14 Acid, sulphuric 06 Ammonia, aqua 23 Ammonium carbonate Ammonium, chloride 55 Ammonium hydrosulphuret 30 Ammonium sulphate 15 Arsenic, white 27 Copper, carbonate, annhy 50 Copper, sulphate 22 Cobalt, sulphate 20 Iron perchloride 40 Lead acetate 35 Nickel ammonium sulphate 25 Nicke Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/canadian-machinery-and-metalworking-january-june-1919-onto-sheets-3-lbs-sq-ft-13-25-18-25-sheets-3-lbs-sq-ft-13-25-18-26sheets-4-to-6-lbs-sq-ft-12-50-12-60cut-sheets-c-per-lb-extracut-sheets-to-size-lc-per-lb-extra-plating-chemicals-acid-boracic-25-acid-hydrochloric-06-acid-nitric-14-acid-sulphuric-06-ammonia-aqua-23-ammonium-carbonate-ammonium-chloride-55-ammonium-hydrosulphuret-30-ammonium-sulphate-15-arsenic-white-27-copper-carbonate-annhy-50-copper-sulphate-22-cobalt-sulphate-20-iron-perchloride-40-lead-acetate-35-nickel-ammonium-sulphate-25-nicke-image336933486.html
. Canadian machinery and metalworking (January-June 1919). onto Sheets, 3 lbs. sq. ft $13 25 $18 25 Sheets, 3% lbs. sq. ft. .. 13 25 18 26Sheets, 4 to 6 lbs. sq. ft. 12 50 12 60Cut sheets, %c per lb. extra.Cut sheets to size, lc per lb. extra. PLATING CHEMICALS. Acid, boracic $ 25 Acid, hydrochloric 06 Acid, nitric 14 Acid, sulphuric 06 Ammonia, aqua 23 Ammonium carbonate Ammonium, chloride 55 Ammonium hydrosulphuret 30 Ammonium sulphate 15 Arsenic, white 27 Copper, carbonate, annhy 50 Copper, sulphate 22 Cobalt, sulphate 20 Iron perchloride 40 Lead acetate 35 Nickel ammonium sulphate 25 Nicke Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/canadian-machinery-and-metalworking-january-june-1919-onto-sheets-3-lbs-sq-ft-13-25-18-25-sheets-3-lbs-sq-ft-13-25-18-26sheets-4-to-6-lbs-sq-ft-12-50-12-60cut-sheets-c-per-lb-extracut-sheets-to-size-lc-per-lb-extra-plating-chemicals-acid-boracic-25-acid-hydrochloric-06-acid-nitric-14-acid-sulphuric-06-ammonia-aqua-23-ammonium-carbonate-ammonium-chloride-55-ammonium-hydrosulphuret-30-ammonium-sulphate-15-arsenic-white-27-copper-carbonate-annhy-50-copper-sulphate-22-cobalt-sulphate-20-iron-perchloride-40-lead-acetate-35-nickel-ammonium-sulphate-25-nicke-image336933486.htmlRM2AG4J2P–. Canadian machinery and metalworking (January-June 1919). onto Sheets, 3 lbs. sq. ft $13 25 $18 25 Sheets, 3% lbs. sq. ft. .. 13 25 18 26Sheets, 4 to 6 lbs. sq. ft. 12 50 12 60Cut sheets, %c per lb. extra.Cut sheets to size, lc per lb. extra. PLATING CHEMICALS. Acid, boracic $ 25 Acid, hydrochloric 06 Acid, nitric 14 Acid, sulphuric 06 Ammonia, aqua 23 Ammonium carbonate Ammonium, chloride 55 Ammonium hydrosulphuret 30 Ammonium sulphate 15 Arsenic, white 27 Copper, carbonate, annhy 50 Copper, sulphate 22 Cobalt, sulphate 20 Iron perchloride 40 Lead acetate 35 Nickel ammonium sulphate 25 Nicke
 . Canadian machinery and metalworking (January-June 1919). o Sheets, 3 lbs. sq. ft $13 25 $13 25 Sheets, 3% lbs. sq. ft. .. 13 25 13 26Sheets, 4 to 6 lbs. sq. ft. 12 50 12 60Cut sheets, %c per lb. extra.Cut sheets to size, lc per lb. extra.. PLATING CHEMICALS. Acid, boracic $ .25 Acid, hydrochloric 06 Acid, nitric 14 Acid, sulphuric 06 Ammonia, aqua 23 Ammonium carbonate Ammonium, chloride 55 Ammonium hydrosulphuret 30 Ammonium sulphate 15 Arsenic, white 27 Copper, carbonate, annhy 50 Copper, sulphate 22 Cobalt, sulphate 20 Iron perchloride 40 Lead acetate 35 Nickel ammonium sulphate 25 Nickel Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/canadian-machinery-and-metalworking-january-june-1919-o-sheets-3-lbs-sq-ft-13-25-13-25-sheets-3-lbs-sq-ft-13-25-13-26sheets-4-to-6-lbs-sq-ft-12-50-12-60cut-sheets-c-per-lb-extracut-sheets-to-size-lc-per-lb-extra-plating-chemicals-acid-boracic-25-acid-hydrochloric-06-acid-nitric-14-acid-sulphuric-06-ammonia-aqua-23-ammonium-carbonate-ammonium-chloride-55-ammonium-hydrosulphuret-30-ammonium-sulphate-15-arsenic-white-27-copper-carbonate-annhy-50-copper-sulphate-22-cobalt-sulphate-20-iron-perchloride-40-lead-acetate-35-nickel-ammonium-sulphate-25-nickel-image336668626.html
. Canadian machinery and metalworking (January-June 1919). o Sheets, 3 lbs. sq. ft $13 25 $13 25 Sheets, 3% lbs. sq. ft. .. 13 25 13 26Sheets, 4 to 6 lbs. sq. ft. 12 50 12 60Cut sheets, %c per lb. extra.Cut sheets to size, lc per lb. extra.. PLATING CHEMICALS. Acid, boracic $ .25 Acid, hydrochloric 06 Acid, nitric 14 Acid, sulphuric 06 Ammonia, aqua 23 Ammonium carbonate Ammonium, chloride 55 Ammonium hydrosulphuret 30 Ammonium sulphate 15 Arsenic, white 27 Copper, carbonate, annhy 50 Copper, sulphate 22 Cobalt, sulphate 20 Iron perchloride 40 Lead acetate 35 Nickel ammonium sulphate 25 Nickel Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/canadian-machinery-and-metalworking-january-june-1919-o-sheets-3-lbs-sq-ft-13-25-13-25-sheets-3-lbs-sq-ft-13-25-13-26sheets-4-to-6-lbs-sq-ft-12-50-12-60cut-sheets-c-per-lb-extracut-sheets-to-size-lc-per-lb-extra-plating-chemicals-acid-boracic-25-acid-hydrochloric-06-acid-nitric-14-acid-sulphuric-06-ammonia-aqua-23-ammonium-carbonate-ammonium-chloride-55-ammonium-hydrosulphuret-30-ammonium-sulphate-15-arsenic-white-27-copper-carbonate-annhy-50-copper-sulphate-22-cobalt-sulphate-20-iron-perchloride-40-lead-acetate-35-nickel-ammonium-sulphate-25-nickel-image336668626.htmlRM2AFMG7E–. Canadian machinery and metalworking (January-June 1919). o Sheets, 3 lbs. sq. ft $13 25 $13 25 Sheets, 3% lbs. sq. ft. .. 13 25 13 26Sheets, 4 to 6 lbs. sq. ft. 12 50 12 60Cut sheets, %c per lb. extra.Cut sheets to size, lc per lb. extra.. PLATING CHEMICALS. Acid, boracic $ .25 Acid, hydrochloric 06 Acid, nitric 14 Acid, sulphuric 06 Ammonia, aqua 23 Ammonium carbonate Ammonium, chloride 55 Ammonium hydrosulphuret 30 Ammonium sulphate 15 Arsenic, white 27 Copper, carbonate, annhy 50 Copper, sulphate 22 Cobalt, sulphate 20 Iron perchloride 40 Lead acetate 35 Nickel ammonium sulphate 25 Nickel
 Practical physiological chemistry; a book designed for use in courses in practical physiological chemistry in schools of medicine and of science . acid (as KSCN) 0-15 o.or Indican o.oi O.OOI Ammonia 0.65 0.04 1 Sodium chloride 16.5 I.I i Phosphoric acid 2-5 0-15 Total sulphuric acid^ 2-5 1 Silicic acid 0.45 0.03 Potassium (K2O) 2-5 0.15 i 1 Sodium (NajO) 5-0 0-3 Calcium (CaO) 0.25 0-015 Iron • o. 005 o. 0004 1 For data as to partition of sulphur and nitrogen, see Chapter XXVII on Metabolism. 372 PHYSIOLOGICAL CHEMISTRY NHoUREA, C = 0. NHo Urea is the principal end-product of the metaboHsm of p Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/practical-physiological-chemistry-a-book-designed-for-use-in-courses-in-practical-physiological-chemistry-in-schools-of-medicine-and-of-science-acid-as-kscn-0-15-oor-indican-ooi-oooi-ammonia-065-004-1-sodium-chloride-165-ii-i-phosphoric-acid-2-5-0-15-total-sulphuric-acid-2-5-1-silicic-acid-045-003-potassium-k2o-2-5-015-i-1-sodium-najo-5-0-0-3-calcium-cao-025-0-015-iron-o-005-o-0004-1-for-data-as-to-partition-of-sulphur-and-nitrogen-see-chapter-xxvii-on-metabolism-372-physiological-chemistry-nhourea-c-=-0-nho-urea-is-the-principal-end-product-of-the-metabohsm-of-p-image340131867.html
Practical physiological chemistry; a book designed for use in courses in practical physiological chemistry in schools of medicine and of science . acid (as KSCN) 0-15 o.or Indican o.oi O.OOI Ammonia 0.65 0.04 1 Sodium chloride 16.5 I.I i Phosphoric acid 2-5 0-15 Total sulphuric acid^ 2-5 1 Silicic acid 0.45 0.03 Potassium (K2O) 2-5 0.15 i 1 Sodium (NajO) 5-0 0-3 Calcium (CaO) 0.25 0-015 Iron • o. 005 o. 0004 1 For data as to partition of sulphur and nitrogen, see Chapter XXVII on Metabolism. 372 PHYSIOLOGICAL CHEMISTRY NHoUREA, C = 0. NHo Urea is the principal end-product of the metaboHsm of p Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/practical-physiological-chemistry-a-book-designed-for-use-in-courses-in-practical-physiological-chemistry-in-schools-of-medicine-and-of-science-acid-as-kscn-0-15-oor-indican-ooi-oooi-ammonia-065-004-1-sodium-chloride-165-ii-i-phosphoric-acid-2-5-0-15-total-sulphuric-acid-2-5-1-silicic-acid-045-003-potassium-k2o-2-5-015-i-1-sodium-najo-5-0-0-3-calcium-cao-025-0-015-iron-o-005-o-0004-1-for-data-as-to-partition-of-sulphur-and-nitrogen-see-chapter-xxvii-on-metabolism-372-physiological-chemistry-nhourea-c-=-0-nho-urea-is-the-principal-end-product-of-the-metabohsm-of-p-image340131867.htmlRM2ANA9JK–Practical physiological chemistry; a book designed for use in courses in practical physiological chemistry in schools of medicine and of science . acid (as KSCN) 0-15 o.or Indican o.oi O.OOI Ammonia 0.65 0.04 1 Sodium chloride 16.5 I.I i Phosphoric acid 2-5 0-15 Total sulphuric acid^ 2-5 1 Silicic acid 0.45 0.03 Potassium (K2O) 2-5 0.15 i 1 Sodium (NajO) 5-0 0-3 Calcium (CaO) 0.25 0-015 Iron • o. 005 o. 0004 1 For data as to partition of sulphur and nitrogen, see Chapter XXVII on Metabolism. 372 PHYSIOLOGICAL CHEMISTRY NHoUREA, C = 0. NHo Urea is the principal end-product of the metaboHsm of p
 . Mechanical appliances, mechanical movements and novelties of construction; a complete work and a continuation, as a second volume, of the author's book entitled 'Mechanical movements, powers and devices' ... including an explanatory chapter on the leading conceptions of perpetual motion existing during the past three centuries. acid liquor, circu-lated by pumps, containing sulphate of ammonia with about 4 per centexcess of free sulphuric acid. Combination of the ammonia of the gas with the free acid takesplace, giving still more sulphate of ammonia, so that to make theprocess continuous, som Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/mechanical-appliances-mechanical-movements-and-novelties-of-construction-a-complete-work-and-a-continuation-as-a-second-volume-of-the-authors-book-entitled-mechanical-movements-powers-and-devices-including-an-explanatory-chapter-on-the-leading-conceptions-of-perpetual-motion-existing-during-the-past-three-centuries-acid-liquor-circu-lated-by-pumps-containing-sulphate-of-ammonia-with-about-4-per-centexcess-of-free-sulphuric-acid-combination-of-the-ammonia-of-the-gas-with-the-free-acid-takesplace-giving-still-more-sulphate-of-ammonia-so-that-to-make-theprocess-continuous-som-image370382676.html
. Mechanical appliances, mechanical movements and novelties of construction; a complete work and a continuation, as a second volume, of the author's book entitled 'Mechanical movements, powers and devices' ... including an explanatory chapter on the leading conceptions of perpetual motion existing during the past three centuries. acid liquor, circu-lated by pumps, containing sulphate of ammonia with about 4 per centexcess of free sulphuric acid. Combination of the ammonia of the gas with the free acid takesplace, giving still more sulphate of ammonia, so that to make theprocess continuous, som Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/mechanical-appliances-mechanical-movements-and-novelties-of-construction-a-complete-work-and-a-continuation-as-a-second-volume-of-the-authors-book-entitled-mechanical-movements-powers-and-devices-including-an-explanatory-chapter-on-the-leading-conceptions-of-perpetual-motion-existing-during-the-past-three-centuries-acid-liquor-circu-lated-by-pumps-containing-sulphate-of-ammonia-with-about-4-per-centexcess-of-free-sulphuric-acid-combination-of-the-ammonia-of-the-gas-with-the-free-acid-takesplace-giving-still-more-sulphate-of-ammonia-so-that-to-make-theprocess-continuous-som-image370382676.htmlRM2CEGATM–. Mechanical appliances, mechanical movements and novelties of construction; a complete work and a continuation, as a second volume, of the author's book entitled 'Mechanical movements, powers and devices' ... including an explanatory chapter on the leading conceptions of perpetual motion existing during the past three centuries. acid liquor, circu-lated by pumps, containing sulphate of ammonia with about 4 per centexcess of free sulphuric acid. Combination of the ammonia of the gas with the free acid takesplace, giving still more sulphate of ammonia, so that to make theprocess continuous, som
 . Essentials of laboratory diagnosis; designed for students and practitioners. he end of this timethe bell-jar is removed, the acid titrated with tenth-normalsodium hydrate, and the number of cubic centimeters of re-maining acid determined. One cubic centimeter of tenth-nor-mal sulphuric acid neutralized by the evolved ammonia repre-sents 0.001704 gram of ammonia. This figure is multiplied by4 to obtain the percentage ammonia value. If any moisture ispresent on the inside of the bell-jar it should be washed into thesulphuric acid before titration.6 5 Websters Diagnosis. 6 Ibid. AMMONIA. 257 Fo Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/essentials-of-laboratory-diagnosis-designed-for-students-and-practitioners-he-end-of-this-timethe-bell-jar-is-removed-the-acid-titrated-with-tenth-normalsodium-hydrate-and-the-number-of-cubic-centimeters-of-re-maining-acid-determined-one-cubic-centimeter-of-tenth-nor-mal-sulphuric-acid-neutralized-by-the-evolved-ammonia-repre-sents-0001704-gram-of-ammonia-this-figure-is-multiplied-by4-to-obtain-the-percentage-ammonia-value-if-any-moisture-ispresent-on-the-inside-of-the-bell-jar-it-should-be-washed-into-thesulphuric-acid-before-titration6-5-websters-diagnosis-6-ibid-ammonia-257-fo-image370398122.html
. Essentials of laboratory diagnosis; designed for students and practitioners. he end of this timethe bell-jar is removed, the acid titrated with tenth-normalsodium hydrate, and the number of cubic centimeters of re-maining acid determined. One cubic centimeter of tenth-nor-mal sulphuric acid neutralized by the evolved ammonia repre-sents 0.001704 gram of ammonia. This figure is multiplied by4 to obtain the percentage ammonia value. If any moisture ispresent on the inside of the bell-jar it should be washed into thesulphuric acid before titration.6 5 Websters Diagnosis. 6 Ibid. AMMONIA. 257 Fo Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/essentials-of-laboratory-diagnosis-designed-for-students-and-practitioners-he-end-of-this-timethe-bell-jar-is-removed-the-acid-titrated-with-tenth-normalsodium-hydrate-and-the-number-of-cubic-centimeters-of-re-maining-acid-determined-one-cubic-centimeter-of-tenth-nor-mal-sulphuric-acid-neutralized-by-the-evolved-ammonia-repre-sents-0001704-gram-of-ammonia-this-figure-is-multiplied-by4-to-obtain-the-percentage-ammonia-value-if-any-moisture-ispresent-on-the-inside-of-the-bell-jar-it-should-be-washed-into-thesulphuric-acid-before-titration6-5-websters-diagnosis-6-ibid-ammonia-257-fo-image370398122.htmlRM2CEH2GA–. Essentials of laboratory diagnosis; designed for students and practitioners. he end of this timethe bell-jar is removed, the acid titrated with tenth-normalsodium hydrate, and the number of cubic centimeters of re-maining acid determined. One cubic centimeter of tenth-nor-mal sulphuric acid neutralized by the evolved ammonia repre-sents 0.001704 gram of ammonia. This figure is multiplied by4 to obtain the percentage ammonia value. If any moisture ispresent on the inside of the bell-jar it should be washed into thesulphuric acid before titration.6 5 Websters Diagnosis. 6 Ibid. AMMONIA. 257 Fo
 . The Gardeners' chronicle and agricultural gazette . is such as to need8 ounces of sulphuric aciu for its saturation. If 1 cwt. ofsulphuric acid will saturate ammonia enough for a strongdressing for 1 aci-e of Grass, then 200 gallons of the liquorshould suffice for an acre. It had better be diluted withthree times its quantity of water before application, or itshould be poured over 8 or 10 cubic yards of earth beforeapplication—or it may be applied during wet weather. Measurement OF Timber : Woodman. Blackies AgriculturalCalculator. Messrs. Suttons* Trial Ground.—Messrs. Sutton write asf- tlo Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/the-gardeners-chronicle-and-agricultural-gazette-is-such-as-to-need8-ounces-of-sulphuric-aciu-for-its-saturation-if-1-cwt-ofsulphuric-acid-will-saturate-ammonia-enough-for-a-strongdressing-for-1-aci-e-of-grass-then-200-gallons-of-the-liquorshould-suffice-for-an-acre-it-had-better-be-diluted-withthree-times-its-quantity-of-water-before-application-or-itshould-be-poured-over-8-or-10-cubic-yards-of-earth-beforeapplicationor-it-may-be-applied-during-wet-weather-measurement-of-timber-woodman-blackies-agriculturalcalculator-messrs-suttons-trial-groundmessrs-sutton-write-asf-tlo-image369813187.html
. The Gardeners' chronicle and agricultural gazette . is such as to need8 ounces of sulphuric aciu for its saturation. If 1 cwt. ofsulphuric acid will saturate ammonia enough for a strongdressing for 1 aci-e of Grass, then 200 gallons of the liquorshould suffice for an acre. It had better be diluted withthree times its quantity of water before application, or itshould be poured over 8 or 10 cubic yards of earth beforeapplication—or it may be applied during wet weather. Measurement OF Timber : Woodman. Blackies AgriculturalCalculator. Messrs. Suttons* Trial Ground.—Messrs. Sutton write asf- tlo Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/the-gardeners-chronicle-and-agricultural-gazette-is-such-as-to-need8-ounces-of-sulphuric-aciu-for-its-saturation-if-1-cwt-ofsulphuric-acid-will-saturate-ammonia-enough-for-a-strongdressing-for-1-aci-e-of-grass-then-200-gallons-of-the-liquorshould-suffice-for-an-acre-it-had-better-be-diluted-withthree-times-its-quantity-of-water-before-application-or-itshould-be-poured-over-8-or-10-cubic-yards-of-earth-beforeapplicationor-it-may-be-applied-during-wet-weather-measurement-of-timber-woodman-blackies-agriculturalcalculator-messrs-suttons-trial-groundmessrs-sutton-write-asf-tlo-image369813187.htmlRM2CDJCDR–. The Gardeners' chronicle and agricultural gazette . is such as to need8 ounces of sulphuric aciu for its saturation. If 1 cwt. ofsulphuric acid will saturate ammonia enough for a strongdressing for 1 aci-e of Grass, then 200 gallons of the liquorshould suffice for an acre. It had better be diluted withthree times its quantity of water before application, or itshould be poured over 8 or 10 cubic yards of earth beforeapplication—or it may be applied during wet weather. Measurement OF Timber : Woodman. Blackies AgriculturalCalculator. Messrs. Suttons* Trial Ground.—Messrs. Sutton write asf- tlo
 . Railway mechanical engineer . When we consider that uric acid and ammonia are depositedon stock carrv-ing equipment, and sulphuric acids on coal carry-ing vehicles, and that sulphur fumes from coal-burning loco-motives are present on all railway equipment, it is apparent thatan oil which will absorb a higli percentage of moisture willcarry these destructive acids into the paint next to the metaland accelerate its destruction. To determine what would make tlie most impermeal)levehicle,* I made the following tests: Exhibit A was composed of films of treated and non-treatedlinseed and other oil Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/railway-mechanical-engineer-when-we-consider-that-uric-acid-and-ammonia-are-depositedon-stock-carrv-ing-equipment-and-sulphuric-acids-on-coal-carry-ing-vehicles-and-that-sulphur-fumes-from-coal-burning-loco-motives-are-present-on-all-railway-equipment-it-is-apparent-thatan-oil-which-will-absorb-a-higli-percentage-of-moisture-willcarry-these-destructive-acids-into-the-paint-next-to-the-metaland-accelerate-its-destruction-to-determine-what-would-make-tlie-most-impermeallevehicle-i-made-the-following-tests-exhibit-a-was-composed-of-films-of-treated-and-non-treatedlinseed-and-other-oil-image371818607.html
. Railway mechanical engineer . When we consider that uric acid and ammonia are depositedon stock carrv-ing equipment, and sulphuric acids on coal carry-ing vehicles, and that sulphur fumes from coal-burning loco-motives are present on all railway equipment, it is apparent thatan oil which will absorb a higli percentage of moisture willcarry these destructive acids into the paint next to the metaland accelerate its destruction. To determine what would make tlie most impermeal)levehicle,* I made the following tests: Exhibit A was composed of films of treated and non-treatedlinseed and other oil Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/railway-mechanical-engineer-when-we-consider-that-uric-acid-and-ammonia-are-depositedon-stock-carrv-ing-equipment-and-sulphuric-acids-on-coal-carry-ing-vehicles-and-that-sulphur-fumes-from-coal-burning-loco-motives-are-present-on-all-railway-equipment-it-is-apparent-thatan-oil-which-will-absorb-a-higli-percentage-of-moisture-willcarry-these-destructive-acids-into-the-paint-next-to-the-metaland-accelerate-its-destruction-to-determine-what-would-make-tlie-most-impermeallevehicle-i-made-the-following-tests-exhibit-a-was-composed-of-films-of-treated-and-non-treatedlinseed-and-other-oil-image371818607.htmlRM2CGWPBY–. Railway mechanical engineer . When we consider that uric acid and ammonia are depositedon stock carrv-ing equipment, and sulphuric acids on coal carry-ing vehicles, and that sulphur fumes from coal-burning loco-motives are present on all railway equipment, it is apparent thatan oil which will absorb a higli percentage of moisture willcarry these destructive acids into the paint next to the metaland accelerate its destruction. To determine what would make tlie most impermeal)levehicle,* I made the following tests: Exhibit A was composed of films of treated and non-treatedlinseed and other oil
![. Principles of agricultural chemistry [microform] . Fig. 70. —Nitrate of soda, partly blasted up. plants, and, unless taken up by plants, will be washed from thesoil. It contains about 15 per cent, of nitrogen. It is oftencalled chile saltpeter. Sulphate of ammonia is a by-product obtained in the manu-facture from coal, of illuminating gas, and of coke. A part of thenitrogen of the coal passes off as ammonia, and is removed bypassing the gas through sulphuric acid, forming sulphate of am-monia (NH^)2S04. It contains about 20 per cent, of nitrogen.Ammonia is fixed by the soil, and is not as av Stock Photo . Principles of agricultural chemistry [microform] . Fig. 70. —Nitrate of soda, partly blasted up. plants, and, unless taken up by plants, will be washed from thesoil. It contains about 15 per cent, of nitrogen. It is oftencalled chile saltpeter. Sulphate of ammonia is a by-product obtained in the manu-facture from coal, of illuminating gas, and of coke. A part of thenitrogen of the coal passes off as ammonia, and is removed bypassing the gas through sulphuric acid, forming sulphate of am-monia (NH^)2S04. It contains about 20 per cent, of nitrogen.Ammonia is fixed by the soil, and is not as av Stock Photo](https://c8.alamy.com/comp/2CHAYW2/principles-of-agricultural-chemistry-microform-fig-70-nitrate-of-soda-partly-blasted-up-plants-and-unless-taken-up-by-plants-will-be-washed-from-thesoil-it-contains-about-15-per-cent-of-nitrogen-it-is-oftencalled-chile-saltpeter-sulphate-of-ammonia-is-a-by-product-obtained-in-the-manu-facture-from-coal-of-illuminating-gas-and-of-coke-a-part-of-thenitrogen-of-the-coal-passes-off-as-ammonia-and-is-removed-bypassing-the-gas-through-sulphuric-acid-forming-sulphate-of-am-monia-nh2s04-it-contains-about-20-per-cent-of-nitrogenammonia-is-fixed-by-the-soil-and-is-not-as-av-2CHAYW2.jpg) . Principles of agricultural chemistry [microform] . Fig. 70. —Nitrate of soda, partly blasted up. plants, and, unless taken up by plants, will be washed from thesoil. It contains about 15 per cent, of nitrogen. It is oftencalled chile saltpeter. Sulphate of ammonia is a by-product obtained in the manu-facture from coal, of illuminating gas, and of coke. A part of thenitrogen of the coal passes off as ammonia, and is removed bypassing the gas through sulphuric acid, forming sulphate of am-monia (NH^)2S04. It contains about 20 per cent, of nitrogen.Ammonia is fixed by the soil, and is not as av Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/principles-of-agricultural-chemistry-microform-fig-70-nitrate-of-soda-partly-blasted-up-plants-and-unless-taken-up-by-plants-will-be-washed-from-thesoil-it-contains-about-15-per-cent-of-nitrogen-it-is-oftencalled-chile-saltpeter-sulphate-of-ammonia-is-a-by-product-obtained-in-the-manu-facture-from-coal-of-illuminating-gas-and-of-coke-a-part-of-thenitrogen-of-the-coal-passes-off-as-ammonia-and-is-removed-bypassing-the-gas-through-sulphuric-acid-forming-sulphate-of-am-monia-nh2s04-it-contains-about-20-per-cent-of-nitrogenammonia-is-fixed-by-the-soil-and-is-not-as-av-image372108270.html
. Principles of agricultural chemistry [microform] . Fig. 70. —Nitrate of soda, partly blasted up. plants, and, unless taken up by plants, will be washed from thesoil. It contains about 15 per cent, of nitrogen. It is oftencalled chile saltpeter. Sulphate of ammonia is a by-product obtained in the manu-facture from coal, of illuminating gas, and of coke. A part of thenitrogen of the coal passes off as ammonia, and is removed bypassing the gas through sulphuric acid, forming sulphate of am-monia (NH^)2S04. It contains about 20 per cent, of nitrogen.Ammonia is fixed by the soil, and is not as av Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/principles-of-agricultural-chemistry-microform-fig-70-nitrate-of-soda-partly-blasted-up-plants-and-unless-taken-up-by-plants-will-be-washed-from-thesoil-it-contains-about-15-per-cent-of-nitrogen-it-is-oftencalled-chile-saltpeter-sulphate-of-ammonia-is-a-by-product-obtained-in-the-manu-facture-from-coal-of-illuminating-gas-and-of-coke-a-part-of-thenitrogen-of-the-coal-passes-off-as-ammonia-and-is-removed-bypassing-the-gas-through-sulphuric-acid-forming-sulphate-of-am-monia-nh2s04-it-contains-about-20-per-cent-of-nitrogenammonia-is-fixed-by-the-soil-and-is-not-as-av-image372108270.htmlRM2CHAYW2–. Principles of agricultural chemistry [microform] . Fig. 70. —Nitrate of soda, partly blasted up. plants, and, unless taken up by plants, will be washed from thesoil. It contains about 15 per cent, of nitrogen. It is oftencalled chile saltpeter. Sulphate of ammonia is a by-product obtained in the manu-facture from coal, of illuminating gas, and of coke. A part of thenitrogen of the coal passes off as ammonia, and is removed bypassing the gas through sulphuric acid, forming sulphate of am-monia (NH^)2S04. It contains about 20 per cent, of nitrogen.Ammonia is fixed by the soil, and is not as av
 . The Gardeners' Chronicle and Agricultural Gazette . nure,7^ per ton; Superphosphate of Lime, 7^; Sulphuric Acid andCoprolites, 6;.—Office, l,?Adelaide Place, London Bridge. N.B. Genuine Peruvian Guano, guaranteed to contain 16 percuTtt. of ammonia. Nitrate of Soda, Sulphate of Ammonia, andother Chemical Manures. THE LONDON MANURE COMPANY have thefollowing ready for immediate delivery:—CORN MANURES. CONCENTRATEU URATE, for Roots, Grasses, &c.KITRO-PHOSPHATE, or HLOOD MAK LRE.SUPERPHOSPHATE of LIME.PERUVIAN GUANO direct from importers warebonses^Ifcitrate of Soda, Sulphate of Ammonia, and ever Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/the-gardeners-chronicle-and-agricultural-gazette-nure7-per-ton-superphosphate-of-lime-7-sulphuric-acid-andcoprolites-6office-ladelaide-place-london-bridge-nb-genuine-peruvian-guano-guaranteed-to-contain-16-percuttt-of-ammonia-nitrate-of-soda-sulphate-of-ammonia-andother-chemical-manures-the-london-manure-company-have-thefollowing-ready-for-immediate-deliverycorn-manures-concentrateu-urate-for-roots-grasses-ckitro-phosphate-or-hlood-mak-lresuperphosphate-of-limeperuvian-guano-direct-from-importers-warebonsesifcitrate-of-soda-sulphate-of-ammonia-and-ever-image370140878.html
. The Gardeners' Chronicle and Agricultural Gazette . nure,7^ per ton; Superphosphate of Lime, 7^; Sulphuric Acid andCoprolites, 6;.—Office, l,?Adelaide Place, London Bridge. N.B. Genuine Peruvian Guano, guaranteed to contain 16 percuTtt. of ammonia. Nitrate of Soda, Sulphate of Ammonia, andother Chemical Manures. THE LONDON MANURE COMPANY have thefollowing ready for immediate delivery:—CORN MANURES. CONCENTRATEU URATE, for Roots, Grasses, &c.KITRO-PHOSPHATE, or HLOOD MAK LRE.SUPERPHOSPHATE of LIME.PERUVIAN GUANO direct from importers warebonses^Ifcitrate of Soda, Sulphate of Ammonia, and ever Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/the-gardeners-chronicle-and-agricultural-gazette-nure7-per-ton-superphosphate-of-lime-7-sulphuric-acid-andcoprolites-6office-ladelaide-place-london-bridge-nb-genuine-peruvian-guano-guaranteed-to-contain-16-percuttt-of-ammonia-nitrate-of-soda-sulphate-of-ammonia-andother-chemical-manures-the-london-manure-company-have-thefollowing-ready-for-immediate-deliverycorn-manures-concentrateu-urate-for-roots-grasses-ckitro-phosphate-or-hlood-mak-lresuperphosphate-of-limeperuvian-guano-direct-from-importers-warebonsesifcitrate-of-soda-sulphate-of-ammonia-and-ever-image370140878.htmlRM2CE5AD2–. The Gardeners' Chronicle and Agricultural Gazette . nure,7^ per ton; Superphosphate of Lime, 7^; Sulphuric Acid andCoprolites, 6;.—Office, l,?Adelaide Place, London Bridge. N.B. Genuine Peruvian Guano, guaranteed to contain 16 percuTtt. of ammonia. Nitrate of Soda, Sulphate of Ammonia, andother Chemical Manures. THE LONDON MANURE COMPANY have thefollowing ready for immediate delivery:—CORN MANURES. CONCENTRATEU URATE, for Roots, Grasses, &c.KITRO-PHOSPHATE, or HLOOD MAK LRE.SUPERPHOSPHATE of LIME.PERUVIAN GUANO direct from importers warebonses^Ifcitrate of Soda, Sulphate of Ammonia, and ever
 . Sessional papers of the Dominion of Canada 1901 . iderable amountsof it. Doth were straight alum powders, and gave results as follows:— Sample 19,592 (b.) 607 ammonia sulphate. 19,560 (b.) 7-52 Five other samples gave traces of ammonia. ADULTERATION OF FOOD. 73 SESSIONAL PAPER No. 14 The low percentage of sulphuric acid present in many of these powders, togetherwith the fact that recently, imported samples of burnt alum containing only traces ofalkali have come under my notice, leads me to infer that burnt sulphate of alumina istaking the place of burnt alum properly so called, This gives a Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/sessional-papers-of-the-dominion-of-canada-1901-iderable-amountsof-it-doth-were-straight-alum-powders-and-gave-results-as-follows-sample-19592-b-607-ammonia-sulphate-19560-b-7-52-five-other-samples-gave-traces-of-ammonia-adulteration-of-food-73-sessional-paper-no-14-the-low-percentage-of-sulphuric-acid-present-in-many-of-these-powders-togetherwith-the-fact-that-recently-imported-samples-of-burnt-alum-containing-only-traces-ofalkali-have-come-under-my-notice-leads-me-to-infer-that-burnt-sulphate-of-alumina-istaking-the-place-of-burnt-alum-properly-so-called-this-gives-a-image370009895.html
. Sessional papers of the Dominion of Canada 1901 . iderable amountsof it. Doth were straight alum powders, and gave results as follows:— Sample 19,592 (b.) 607 ammonia sulphate. 19,560 (b.) 7-52 Five other samples gave traces of ammonia. ADULTERATION OF FOOD. 73 SESSIONAL PAPER No. 14 The low percentage of sulphuric acid present in many of these powders, togetherwith the fact that recently, imported samples of burnt alum containing only traces ofalkali have come under my notice, leads me to infer that burnt sulphate of alumina istaking the place of burnt alum properly so called, This gives a Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/sessional-papers-of-the-dominion-of-canada-1901-iderable-amountsof-it-doth-were-straight-alum-powders-and-gave-results-as-follows-sample-19592-b-607-ammonia-sulphate-19560-b-7-52-five-other-samples-gave-traces-of-ammonia-adulteration-of-food-73-sessional-paper-no-14-the-low-percentage-of-sulphuric-acid-present-in-many-of-these-powders-togetherwith-the-fact-that-recently-imported-samples-of-burnt-alum-containing-only-traces-ofalkali-have-come-under-my-notice-leads-me-to-infer-that-burnt-sulphate-of-alumina-istaking-the-place-of-burnt-alum-properly-so-called-this-gives-a-image370009895.htmlRM2CDYBB3–. Sessional papers of the Dominion of Canada 1901 . iderable amountsof it. Doth were straight alum powders, and gave results as follows:— Sample 19,592 (b.) 607 ammonia sulphate. 19,560 (b.) 7-52 Five other samples gave traces of ammonia. ADULTERATION OF FOOD. 73 SESSIONAL PAPER No. 14 The low percentage of sulphuric acid present in many of these powders, togetherwith the fact that recently, imported samples of burnt alum containing only traces ofalkali have come under my notice, leads me to infer that burnt sulphate of alumina istaking the place of burnt alum properly so called, This gives a
 . Hardware merchandising January-June 1900. ly dissolved. If it is not, then adda little more cyanide until it is. Amoderately strong circuit of electricity isrequired, and the bath may be either hotor cold. The following is said to be a good warmbath for iron and steel: Acetate of copper, 3 t-5 ounces.Carbonate of soda, 3 1-5 ounces.Bisulphite of of ?oda, 1 1-5 ounces.Cyanide of potassium, 4/s ounces.Aqua ammonia, 1 4-5 ounces.Water, 1 gallon. The following is said to be a good bathfor coating iron articles with copper bydipping : Sulphate of copper, 3% ounces.Sulphuric acid, 3^ ounces.Water, Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/hardware-merchandising-january-june-1900-ly-dissolved-if-it-is-not-then-adda-little-more-cyanide-until-it-is-amoderately-strong-circuit-of-electricity-isrequired-and-the-bath-may-be-either-hotor-cold-the-following-is-said-to-be-a-good-warmbath-for-iron-and-steel-acetate-of-copper-3-t-5-ouncescarbonate-of-soda-3-1-5-ouncesbisulphite-of-of-oda-1-1-5-ouncescyanide-of-potassium-4s-ouncesaqua-ammonia-1-4-5-ounceswater-1-gallon-the-following-is-said-to-be-a-good-bathfor-coating-iron-articles-with-copper-bydipping-sulphate-of-copper-3-ouncessulphuric-acid-3-ounceswater-image370479373.html
. Hardware merchandising January-June 1900. ly dissolved. If it is not, then adda little more cyanide until it is. Amoderately strong circuit of electricity isrequired, and the bath may be either hotor cold. The following is said to be a good warmbath for iron and steel: Acetate of copper, 3 t-5 ounces.Carbonate of soda, 3 1-5 ounces.Bisulphite of of ?oda, 1 1-5 ounces.Cyanide of potassium, 4/s ounces.Aqua ammonia, 1 4-5 ounces.Water, 1 gallon. The following is said to be a good bathfor coating iron articles with copper bydipping : Sulphate of copper, 3% ounces.Sulphuric acid, 3^ ounces.Water, Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/hardware-merchandising-january-june-1900-ly-dissolved-if-it-is-not-then-adda-little-more-cyanide-until-it-is-amoderately-strong-circuit-of-electricity-isrequired-and-the-bath-may-be-either-hotor-cold-the-following-is-said-to-be-a-good-warmbath-for-iron-and-steel-acetate-of-copper-3-t-5-ouncescarbonate-of-soda-3-1-5-ouncesbisulphite-of-of-oda-1-1-5-ouncescyanide-of-potassium-4s-ouncesaqua-ammonia-1-4-5-ounceswater-1-gallon-the-following-is-said-to-be-a-good-bathfor-coating-iron-articles-with-copper-bydipping-sulphate-of-copper-3-ouncessulphuric-acid-3-ounceswater-image370479373.htmlRM2CEMP65–. Hardware merchandising January-June 1900. ly dissolved. If it is not, then adda little more cyanide until it is. Amoderately strong circuit of electricity isrequired, and the bath may be either hotor cold. The following is said to be a good warmbath for iron and steel: Acetate of copper, 3 t-5 ounces.Carbonate of soda, 3 1-5 ounces.Bisulphite of of ?oda, 1 1-5 ounces.Cyanide of potassium, 4/s ounces.Aqua ammonia, 1 4-5 ounces.Water, 1 gallon. The following is said to be a good bathfor coating iron articles with copper bydipping : Sulphate of copper, 3% ounces.Sulphuric acid, 3^ ounces.Water,
 . The coal tar colours of Farbwerke vorm. Meister Lucius & Brüning, Hoechst on Main, Germany and their application in wool dyeing . Commenced with 10—20;o Glaiihcrs s.ill and h „ acetateof ammonia, at 120- 140° K.;the bath is heated slowly tothe boil, kept boilmj; for1/, hours; for dark shades2—4% acetic acid are finallyadded. Dianil Brown G With 10—20% (dauberssalt and 3% sulphuric acid;the goods are entered at120—140° !•., and the bathheated slowly to the boil.. With 10—20°;„ Glauberssalt and 5°/ii acetate of am-monia; the gooils are enteredat 120—140° v.: the bathheated slowly to the boil,a Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/the-coal-tar-colours-of-farbwerke-vorm-meister-lucius-brning-hoechst-on-main-germany-and-their-application-in-wool-dyeing-commenced-with-1020o-glaiihcrs-sill-and-h-acetateof-ammonia-at-120-140-kthe-bath-is-heated-slowly-tothe-boil-kept-boilmj-for1-hours-for-dark-shades24-acetic-acid-are-finallyadded-dianil-brown-g-with-1020-dauberssalt-and-3-sulphuric-acidthe-goods-are-entered-at120140-!-and-the-bathheated-slowly-to-the-boil-with-1020-glauberssalt-and-5ii-acetate-of-am-monia-the-gooils-are-enteredat-120140-v-the-bathheated-slowly-to-the-boila-image371621174.html
. The coal tar colours of Farbwerke vorm. Meister Lucius & Brüning, Hoechst on Main, Germany and their application in wool dyeing . Commenced with 10—20;o Glaiihcrs s.ill and h „ acetateof ammonia, at 120- 140° K.;the bath is heated slowly tothe boil, kept boilmj; for1/, hours; for dark shades2—4% acetic acid are finallyadded. Dianil Brown G With 10—20% (dauberssalt and 3% sulphuric acid;the goods are entered at120—140° !•., and the bathheated slowly to the boil.. With 10—20°;„ Glauberssalt and 5°/ii acetate of am-monia; the gooils are enteredat 120—140° v.: the bathheated slowly to the boil,a Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/the-coal-tar-colours-of-farbwerke-vorm-meister-lucius-brning-hoechst-on-main-germany-and-their-application-in-wool-dyeing-commenced-with-1020o-glaiihcrs-sill-and-h-acetateof-ammonia-at-120-140-kthe-bath-is-heated-slowly-tothe-boil-kept-boilmj-for1-hours-for-dark-shades24-acetic-acid-are-finallyadded-dianil-brown-g-with-1020-dauberssalt-and-3-sulphuric-acidthe-goods-are-entered-at120140-!-and-the-bathheated-slowly-to-the-boil-with-1020-glauberssalt-and-5ii-acetate-of-am-monia-the-gooils-are-enteredat-120140-v-the-bathheated-slowly-to-the-boila-image371621174.htmlRM2CGGPGP–. The coal tar colours of Farbwerke vorm. Meister Lucius & Brüning, Hoechst on Main, Germany and their application in wool dyeing . Commenced with 10—20;o Glaiihcrs s.ill and h „ acetateof ammonia, at 120- 140° K.;the bath is heated slowly tothe boil, kept boilmj; for1/, hours; for dark shades2—4% acetic acid are finallyadded. Dianil Brown G With 10—20% (dauberssalt and 3% sulphuric acid;the goods are entered at120—140° !•., and the bathheated slowly to the boil.. With 10—20°;„ Glauberssalt and 5°/ii acetate of am-monia; the gooils are enteredat 120—140° v.: the bathheated slowly to the boil,a
 . The coal tar colours of Farbwerke vorm. Meister Lucius & Brüning, Hoechst on Main, Germany and their application in wool dyeing . toring of Dyestufts 116. Stoving colours 125. Stoving process 125. Sulphate of Alumina 180. Sulphate of Copper 180. Sulphate of h-on 180. Sulphate of Magnesia 180. Sulphate of Soda ISO. Index. 201 Sulphocyanide of Ammonia 180.Sulphur 180.Sulphuric Acid 180.Sulphurous Acid 182. T. Tables 161 — 196. Tannin 156, 182. Tanning of wool 156. Tartar 136, 183. Tartar Emetic 183. Tartaric Acid 183. Tartar Substitute 183. Thermometric scales, comparison of 195. Tin chloride Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/the-coal-tar-colours-of-farbwerke-vorm-meister-lucius-brning-hoechst-on-main-germany-and-their-application-in-wool-dyeing-toring-of-dyestufts-116-stoving-colours-125-stoving-process-125-sulphate-of-alumina-180-sulphate-of-copper-180-sulphate-of-h-on-180-sulphate-of-magnesia-180-sulphate-of-soda-iso-index-201-sulphocyanide-of-ammonia-180sulphur-180sulphuric-acid-180sulphurous-acid-182-t-tables-161-196-tannin-156-182-tanning-of-wool-156-tartar-136-183-tartar-emetic-183-tartaric-acid-183-tartar-substitute-183-thermometric-scales-comparison-of-195-tin-chloride-image372633865.html
. The coal tar colours of Farbwerke vorm. Meister Lucius & Brüning, Hoechst on Main, Germany and their application in wool dyeing . toring of Dyestufts 116. Stoving colours 125. Stoving process 125. Sulphate of Alumina 180. Sulphate of Copper 180. Sulphate of h-on 180. Sulphate of Magnesia 180. Sulphate of Soda ISO. Index. 201 Sulphocyanide of Ammonia 180.Sulphur 180.Sulphuric Acid 180.Sulphurous Acid 182. T. Tables 161 — 196. Tannin 156, 182. Tanning of wool 156. Tartar 136, 183. Tartar Emetic 183. Tartaric Acid 183. Tartar Substitute 183. Thermometric scales, comparison of 195. Tin chloride Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/the-coal-tar-colours-of-farbwerke-vorm-meister-lucius-brning-hoechst-on-main-germany-and-their-application-in-wool-dyeing-toring-of-dyestufts-116-stoving-colours-125-stoving-process-125-sulphate-of-alumina-180-sulphate-of-copper-180-sulphate-of-h-on-180-sulphate-of-magnesia-180-sulphate-of-soda-iso-index-201-sulphocyanide-of-ammonia-180sulphur-180sulphuric-acid-180sulphurous-acid-182-t-tables-161-196-tannin-156-182-tanning-of-wool-156-tartar-136-183-tartar-emetic-183-tartaric-acid-183-tartar-substitute-183-thermometric-scales-comparison-of-195-tin-chloride-image372633865.htmlRM2CJ6X89–. The coal tar colours of Farbwerke vorm. Meister Lucius & Brüning, Hoechst on Main, Germany and their application in wool dyeing . toring of Dyestufts 116. Stoving colours 125. Stoving process 125. Sulphate of Alumina 180. Sulphate of Copper 180. Sulphate of h-on 180. Sulphate of Magnesia 180. Sulphate of Soda ISO. Index. 201 Sulphocyanide of Ammonia 180.Sulphur 180.Sulphuric Acid 180.Sulphurous Acid 182. T. Tables 161 — 196. Tannin 156, 182. Tanning of wool 156. Tartar 136, 183. Tartar Emetic 183. Tartaric Acid 183. Tartar Substitute 183. Thermometric scales, comparison of 195. Tin chloride
 . The coal tar colours of Farbwerke vorm. Meister Lucius & Brüning, Hoechst on Main, Germany and their application in wool dyeing . Brilliant Crimson O. With 10-20;o r.lanbcrss.ill and 3o sulphuric acid;the g<KKls arc entered at120° I-., the bath heatedto the lioil, and kept boilingfor 1 hour. With 20 /o Glaubers saltand :S,o sulphuric acid; thej;oods arc entered at 120 F-,the bath heated slowly tothe boil and kept boiling for1 hour, l-or goods whichdn not dye through easilythe bath is prepared withformic acid, acetic acid oracetate of ammonia, insteadof with sulphuric acid, andis exhausted Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/the-coal-tar-colours-of-farbwerke-vorm-meister-lucius-brning-hoechst-on-main-germany-and-their-application-in-wool-dyeing-brilliant-crimson-o-with-10-20o-rlanbcrssill-and-3o-sulphuric-acidthe-gltkkls-arc-entered-at120-i-the-bath-heatedto-the-lioil-and-kept-boilingfor-1-hour-with-20-o-glaubers-saltand-so-sulphuric-acid-thejoods-arc-entered-at-120-f-the-bath-heated-slowly-tothe-boil-and-kept-boiling-for1-hour-l-or-goods-whichdn-not-dye-through-easilythe-bath-is-prepared-withformic-acid-acetic-acid-oracetate-of-ammonia-insteadof-with-sulphuric-acid-andis-exhausted-image371621711.html
. The coal tar colours of Farbwerke vorm. Meister Lucius & Brüning, Hoechst on Main, Germany and their application in wool dyeing . Brilliant Crimson O. With 10-20;o r.lanbcrss.ill and 3o sulphuric acid;the g<KKls arc entered at120° I-., the bath heatedto the lioil, and kept boilingfor 1 hour. With 20 /o Glaubers saltand :S,o sulphuric acid; thej;oods arc entered at 120 F-,the bath heated slowly tothe boil and kept boiling for1 hour, l-or goods whichdn not dye through easilythe bath is prepared withformic acid, acetic acid oracetate of ammonia, insteadof with sulphuric acid, andis exhausted Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/the-coal-tar-colours-of-farbwerke-vorm-meister-lucius-brning-hoechst-on-main-germany-and-their-application-in-wool-dyeing-brilliant-crimson-o-with-10-20o-rlanbcrssill-and-3o-sulphuric-acidthe-gltkkls-arc-entered-at120-i-the-bath-heatedto-the-lioil-and-kept-boilingfor-1-hour-with-20-o-glaubers-saltand-so-sulphuric-acid-thejoods-arc-entered-at-120-f-the-bath-heated-slowly-tothe-boil-and-kept-boiling-for1-hour-l-or-goods-whichdn-not-dye-through-easilythe-bath-is-prepared-withformic-acid-acetic-acid-oracetate-of-ammonia-insteadof-with-sulphuric-acid-andis-exhausted-image371621711.htmlRM2CGGR7Y–. The coal tar colours of Farbwerke vorm. Meister Lucius & Brüning, Hoechst on Main, Germany and their application in wool dyeing . Brilliant Crimson O. With 10-20;o r.lanbcrss.ill and 3o sulphuric acid;the g<KKls arc entered at120° I-., the bath heatedto the lioil, and kept boilingfor 1 hour. With 20 /o Glaubers saltand :S,o sulphuric acid; thej;oods arc entered at 120 F-,the bath heated slowly tothe boil and kept boiling for1 hour, l-or goods whichdn not dye through easilythe bath is prepared withformic acid, acetic acid oracetate of ammonia, insteadof with sulphuric acid, andis exhausted
 . The chemistry of agriculture, for students and farmers. Agricultural chemistry. 214 NITROGENOUS FERTILIZERS In nitrifying, the ammonia is changed to nitric acid, and sulphuric acid is set free. Both acids require bases to neutrahze them, and thus there is twice as much hme or other base needed for this fertihzer as is needed for other nitrog- enous materials undergoing nitrification. Lime is used up very rapidly and acidity results (Section 186). III. NITROGEN AS AMINE OR PROTEIN 160. Cyanamid or Lime Nitrogen.—The utilization of atmospheric nitrogen in the manufacture of fertilizers is succ Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/the-chemistry-of-agriculture-for-students-and-farmers-agricultural-chemistry-214-nitrogenous-fertilizers-in-nitrifying-the-ammonia-is-changed-to-nitric-acid-and-sulphuric-acid-is-set-free-both-acids-require-bases-to-neutrahze-them-and-thus-there-is-twice-as-much-hme-or-other-base-needed-for-this-fertihzer-as-is-needed-for-other-nitrog-enous-materials-undergoing-nitrification-lime-is-used-up-very-rapidly-and-acidity-results-section-186-iii-nitrogen-as-amine-or-protein-160-cyanamid-or-lime-nitrogenthe-utilization-of-atmospheric-nitrogen-in-the-manufacture-of-fertilizers-is-succ-image234988367.html
. The chemistry of agriculture, for students and farmers. Agricultural chemistry. 214 NITROGENOUS FERTILIZERS In nitrifying, the ammonia is changed to nitric acid, and sulphuric acid is set free. Both acids require bases to neutrahze them, and thus there is twice as much hme or other base needed for this fertihzer as is needed for other nitrog- enous materials undergoing nitrification. Lime is used up very rapidly and acidity results (Section 186). III. NITROGEN AS AMINE OR PROTEIN 160. Cyanamid or Lime Nitrogen.—The utilization of atmospheric nitrogen in the manufacture of fertilizers is succ Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/the-chemistry-of-agriculture-for-students-and-farmers-agricultural-chemistry-214-nitrogenous-fertilizers-in-nitrifying-the-ammonia-is-changed-to-nitric-acid-and-sulphuric-acid-is-set-free-both-acids-require-bases-to-neutrahze-them-and-thus-there-is-twice-as-much-hme-or-other-base-needed-for-this-fertihzer-as-is-needed-for-other-nitrog-enous-materials-undergoing-nitrification-lime-is-used-up-very-rapidly-and-acidity-results-section-186-iii-nitrogen-as-amine-or-protein-160-cyanamid-or-lime-nitrogenthe-utilization-of-atmospheric-nitrogen-in-the-manufacture-of-fertilizers-is-succ-image234988367.htmlRMRJ8J1K–. The chemistry of agriculture, for students and farmers. Agricultural chemistry. 214 NITROGENOUS FERTILIZERS In nitrifying, the ammonia is changed to nitric acid, and sulphuric acid is set free. Both acids require bases to neutrahze them, and thus there is twice as much hme or other base needed for this fertihzer as is needed for other nitrog- enous materials undergoing nitrification. Lime is used up very rapidly and acidity results (Section 186). III. NITROGEN AS AMINE OR PROTEIN 160. Cyanamid or Lime Nitrogen.—The utilization of atmospheric nitrogen in the manufacture of fertilizers is succ
 . Carnegie Institution of Washington publication. ELECTRICAL CONDUCTIVITIES, ETC. 9 WATER. All of the water used in this work was purified by the method worked out a number of years ago in this laboratory by Jones and Mackay.* It consisted in distilling the distilled water of the laboratory from chromic acid (potassium dichromate and sulphuric acid), which burned up any organic matter present in the water, and then redistilling the water from barium hydroxide. The sulphuric acid held back all ammonia formed from the organic substances, while the barium hydroxide com- bined all the carbon dioxi Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/carnegie-institution-of-washington-publication-electrical-conductivities-etc-9-water-all-of-the-water-used-in-this-work-was-purified-by-the-method-worked-out-a-number-of-years-ago-in-this-laboratory-by-jones-and-mackay-it-consisted-in-distilling-the-distilled-water-of-the-laboratory-from-chromic-acid-potassium-dichromate-and-sulphuric-acid-which-burned-up-any-organic-matter-present-in-the-water-and-then-redistilling-the-water-from-barium-hydroxide-the-sulphuric-acid-held-back-all-ammonia-formed-from-the-organic-substances-while-the-barium-hydroxide-com-bined-all-the-carbon-dioxi-image233486202.html
. Carnegie Institution of Washington publication. ELECTRICAL CONDUCTIVITIES, ETC. 9 WATER. All of the water used in this work was purified by the method worked out a number of years ago in this laboratory by Jones and Mackay.* It consisted in distilling the distilled water of the laboratory from chromic acid (potassium dichromate and sulphuric acid), which burned up any organic matter present in the water, and then redistilling the water from barium hydroxide. The sulphuric acid held back all ammonia formed from the organic substances, while the barium hydroxide com- bined all the carbon dioxi Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/carnegie-institution-of-washington-publication-electrical-conductivities-etc-9-water-all-of-the-water-used-in-this-work-was-purified-by-the-method-worked-out-a-number-of-years-ago-in-this-laboratory-by-jones-and-mackay-it-consisted-in-distilling-the-distilled-water-of-the-laboratory-from-chromic-acid-potassium-dichromate-and-sulphuric-acid-which-burned-up-any-organic-matter-present-in-the-water-and-then-redistilling-the-water-from-barium-hydroxide-the-sulphuric-acid-held-back-all-ammonia-formed-from-the-organic-substances-while-the-barium-hydroxide-com-bined-all-the-carbon-dioxi-image233486202.htmlRMRFT60X–. Carnegie Institution of Washington publication. ELECTRICAL CONDUCTIVITIES, ETC. 9 WATER. All of the water used in this work was purified by the method worked out a number of years ago in this laboratory by Jones and Mackay.* It consisted in distilling the distilled water of the laboratory from chromic acid (potassium dichromate and sulphuric acid), which burned up any organic matter present in the water, and then redistilling the water from barium hydroxide. The sulphuric acid held back all ammonia formed from the organic substances, while the barium hydroxide com- bined all the carbon dioxi
 . Plant analysis: qualitative and quantitative. Plants. § 97. ESTIMATION OF AMMONIA. 81 § 97. A'nim€nm.^--rA. portion of the aqueous extract (g 92) is mixed with two relumes of alcohol and filtered. To the filtrate and washings calcined magnesia is added, and the ammonia distilled off into a receiver containing a measured quastity of normal sulphuric or hydrochloric acid, every precaution being taJien to avoid loss of, ammonia and spirting of the magnesia mixture into the receiver. The apparatus I use is represented in Pig. 2. The flask A should not be more than half full of magnesia. Fig. 2. Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/plant-analysis-qualitative-and-quantitative-plants-97-estimation-of-ammonia-81-97-animnm-ra-portion-of-the-aqueous-extract-g-92-is-mixed-with-two-relumes-of-alcohol-and-filtered-to-the-filtrate-and-washings-calcined-magnesia-is-added-and-the-ammonia-distilled-off-into-a-receiver-containing-a-measured-quastity-of-normal-sulphuric-or-hydrochloric-acid-every-precaution-being-tajien-to-avoid-loss-of-ammonia-and-spirting-of-the-magnesia-mixture-into-the-receiver-the-apparatus-i-use-is-represented-in-pig-2-the-flask-a-should-not-be-more-than-half-full-of-magnesia-fig-2-image232355162.html
. Plant analysis: qualitative and quantitative. Plants. § 97. ESTIMATION OF AMMONIA. 81 § 97. A'nim€nm.^--rA. portion of the aqueous extract (g 92) is mixed with two relumes of alcohol and filtered. To the filtrate and washings calcined magnesia is added, and the ammonia distilled off into a receiver containing a measured quastity of normal sulphuric or hydrochloric acid, every precaution being taJien to avoid loss of, ammonia and spirting of the magnesia mixture into the receiver. The apparatus I use is represented in Pig. 2. The flask A should not be more than half full of magnesia. Fig. 2. Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/plant-analysis-qualitative-and-quantitative-plants-97-estimation-of-ammonia-81-97-animnm-ra-portion-of-the-aqueous-extract-g-92-is-mixed-with-two-relumes-of-alcohol-and-filtered-to-the-filtrate-and-washings-calcined-magnesia-is-added-and-the-ammonia-distilled-off-into-a-receiver-containing-a-measured-quastity-of-normal-sulphuric-or-hydrochloric-acid-every-precaution-being-tajien-to-avoid-loss-of-ammonia-and-spirting-of-the-magnesia-mixture-into-the-receiver-the-apparatus-i-use-is-represented-in-pig-2-the-flask-a-should-not-be-more-than-half-full-of-magnesia-fig-2-image232355162.htmlRMRE0KAJ–. Plant analysis: qualitative and quantitative. Plants. § 97. ESTIMATION OF AMMONIA. 81 § 97. A'nim€nm.^--rA. portion of the aqueous extract (g 92) is mixed with two relumes of alcohol and filtered. To the filtrate and washings calcined magnesia is added, and the ammonia distilled off into a receiver containing a measured quastity of normal sulphuric or hydrochloric acid, every precaution being taJien to avoid loss of, ammonia and spirting of the magnesia mixture into the receiver. The apparatus I use is represented in Pig. 2. The flask A should not be more than half full of magnesia. Fig. 2.
 . Cassell's popular gardening. Gardening. MANURING IN THEORY AND PRACTICE. ammonia by passing through sulphuric acid, and then thi'ough a sokition of carbonate of soda. These conditions were proved to be adapted for healthy growth by growing plants under exactly the same circumstances, but in a garden soil. The prepared soil was also shown to be suitable for Figs. 4, 5, and 6 represent plants of Wheat, Bar- ley, and Oats grown in prepared soil, and supplied with a known quantity of combined nitrogen beyond that contained in the seed sown. Figs. 4, 5, and 6 show the development of the three gra Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/cassells-popular-gardening-gardening-manuring-in-theory-and-practice-ammonia-by-passing-through-sulphuric-acid-and-then-thiough-a-sokition-of-carbonate-of-soda-these-conditions-were-proved-to-be-adapted-for-healthy-growth-by-growing-plants-under-exactly-the-same-circumstances-but-in-a-garden-soil-the-prepared-soil-was-also-shown-to-be-suitable-for-figs-4-5-and-6-represent-plants-of-wheat-bar-ley-and-oats-grown-in-prepared-soil-and-supplied-with-a-known-quantity-of-combined-nitrogen-beyond-that-contained-in-the-seed-sown-figs-4-5-and-6-show-the-development-of-the-three-gra-image233457783.html
. Cassell's popular gardening. Gardening. MANURING IN THEORY AND PRACTICE. ammonia by passing through sulphuric acid, and then thi'ough a sokition of carbonate of soda. These conditions were proved to be adapted for healthy growth by growing plants under exactly the same circumstances, but in a garden soil. The prepared soil was also shown to be suitable for Figs. 4, 5, and 6 represent plants of Wheat, Bar- ley, and Oats grown in prepared soil, and supplied with a known quantity of combined nitrogen beyond that contained in the seed sown. Figs. 4, 5, and 6 show the development of the three gra Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/cassells-popular-gardening-gardening-manuring-in-theory-and-practice-ammonia-by-passing-through-sulphuric-acid-and-then-thiough-a-sokition-of-carbonate-of-soda-these-conditions-were-proved-to-be-adapted-for-healthy-growth-by-growing-plants-under-exactly-the-same-circumstances-but-in-a-garden-soil-the-prepared-soil-was-also-shown-to-be-suitable-for-figs-4-5-and-6-represent-plants-of-wheat-bar-ley-and-oats-grown-in-prepared-soil-and-supplied-with-a-known-quantity-of-combined-nitrogen-beyond-that-contained-in-the-seed-sown-figs-4-5-and-6-show-the-development-of-the-three-gra-image233457783.htmlRMRFPWNY–. Cassell's popular gardening. Gardening. MANURING IN THEORY AND PRACTICE. ammonia by passing through sulphuric acid, and then thi'ough a sokition of carbonate of soda. These conditions were proved to be adapted for healthy growth by growing plants under exactly the same circumstances, but in a garden soil. The prepared soil was also shown to be suitable for Figs. 4, 5, and 6 represent plants of Wheat, Bar- ley, and Oats grown in prepared soil, and supplied with a known quantity of combined nitrogen beyond that contained in the seed sown. Figs. 4, 5, and 6 show the development of the three gra
 . Charles V. Mapes'. Agricultural machinery. 242 C. V. MAPES ILLUSTKATED CATALOGUE. Mapes' Nitrogenized Snper-Phosphate of Lime, For Corn, Cotton, Potatoes, Tobacco, Grain Crops, Vegetable Gardens, Lawns, etc. Composed of Dried Blood, Burnt Bones, Sulphuric Acid, Peruvian Guano, and Sulphate of Ammonia. PATEISTTED 1859. LARGE SILVER MEDAL Awarded by the. AMERICAN INSTITUTE OF NEW YORK. 1859. Testimonials from hundreds of farmers of high standing, who have used it for several years. Does not exhaust the land like guano, but permanently improves it. One hundred pounds of Nitrogenized Super-Phosp Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/charles-v-mapes-agricultural-machinery-242-c-v-mapes-illustkated-catalogue-mapes-nitrogenized-snper-phosphate-of-lime-for-corn-cotton-potatoes-tobacco-grain-crops-vegetable-gardens-lawns-etc-composed-of-dried-blood-burnt-bones-sulphuric-acid-peruvian-guano-and-sulphate-of-ammonia-pateistted-1859-large-silver-medal-awarded-by-the-american-institute-of-new-york-1859-testimonials-from-hundreds-of-farmers-of-high-standing-who-have-used-it-for-several-years-does-not-exhaust-the-land-like-guano-but-permanently-improves-it-one-hundred-pounds-of-nitrogenized-super-phosp-image235061647.html
. Charles V. Mapes'. Agricultural machinery. 242 C. V. MAPES ILLUSTKATED CATALOGUE. Mapes' Nitrogenized Snper-Phosphate of Lime, For Corn, Cotton, Potatoes, Tobacco, Grain Crops, Vegetable Gardens, Lawns, etc. Composed of Dried Blood, Burnt Bones, Sulphuric Acid, Peruvian Guano, and Sulphate of Ammonia. PATEISTTED 1859. LARGE SILVER MEDAL Awarded by the. AMERICAN INSTITUTE OF NEW YORK. 1859. Testimonials from hundreds of farmers of high standing, who have used it for several years. Does not exhaust the land like guano, but permanently improves it. One hundred pounds of Nitrogenized Super-Phosp Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/charles-v-mapes-agricultural-machinery-242-c-v-mapes-illustkated-catalogue-mapes-nitrogenized-snper-phosphate-of-lime-for-corn-cotton-potatoes-tobacco-grain-crops-vegetable-gardens-lawns-etc-composed-of-dried-blood-burnt-bones-sulphuric-acid-peruvian-guano-and-sulphate-of-ammonia-pateistted-1859-large-silver-medal-awarded-by-the-american-institute-of-new-york-1859-testimonials-from-hundreds-of-farmers-of-high-standing-who-have-used-it-for-several-years-does-not-exhaust-the-land-like-guano-but-permanently-improves-it-one-hundred-pounds-of-nitrogenized-super-phosp-image235061647.htmlRMRJBYER–. Charles V. Mapes'. Agricultural machinery. 242 C. V. MAPES ILLUSTKATED CATALOGUE. Mapes' Nitrogenized Snper-Phosphate of Lime, For Corn, Cotton, Potatoes, Tobacco, Grain Crops, Vegetable Gardens, Lawns, etc. Composed of Dried Blood, Burnt Bones, Sulphuric Acid, Peruvian Guano, and Sulphate of Ammonia. PATEISTTED 1859. LARGE SILVER MEDAL Awarded by the. AMERICAN INSTITUTE OF NEW YORK. 1859. Testimonials from hundreds of farmers of high standing, who have used it for several years. Does not exhaust the land like guano, but permanently improves it. One hundred pounds of Nitrogenized Super-Phosp
 . The Cottage gardener. Gardening; Gardening. THE COTTAGE GAKDENER.—ADVERTISEMENTS. rrilE LONDON MANURE COM- J- PANV beg to offer Jis under:— Corn Manure, most valuable for spring drees- ing, Concentrated Urate, Super-Phosphate of Lime, Nitrate of Soda, Sulphate of Ammonia, Fishery and Agricultural Salt, Gypsum, Fossil Bones, Sulphuric Acid, and every other artificial manure ; also, a constant supply of English and Foreign Linseed Cake, Peruvian Guano, guaranteed the genuine im- portation of Messrs. A. Gibbs and Sons, ^g lOs per ton, or ^Q 5s in quantities of five tons or upwards. EDWARD PURSE Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/the-cottage-gardener-gardening-gardening-the-cottage-gakdeneradvertisements-rrile-london-manure-com-j-panv-beg-to-offer-jis-under-corn-manure-most-valuable-for-spring-drees-ing-concentrated-urate-super-phosphate-of-lime-nitrate-of-soda-sulphate-of-ammonia-fishery-and-agricultural-salt-gypsum-fossil-bones-sulphuric-acid-and-every-other-artificial-manure-also-a-constant-supply-of-english-and-foreign-linseed-cake-peruvian-guano-guaranteed-the-genuine-im-portation-of-messrs-a-gibbs-and-sons-g-los-per-ton-or-q-5s-in-quantities-of-five-tons-or-upwards-edward-purse-image232499250.html
. The Cottage gardener. Gardening; Gardening. THE COTTAGE GAKDENER.—ADVERTISEMENTS. rrilE LONDON MANURE COM- J- PANV beg to offer Jis under:— Corn Manure, most valuable for spring drees- ing, Concentrated Urate, Super-Phosphate of Lime, Nitrate of Soda, Sulphate of Ammonia, Fishery and Agricultural Salt, Gypsum, Fossil Bones, Sulphuric Acid, and every other artificial manure ; also, a constant supply of English and Foreign Linseed Cake, Peruvian Guano, guaranteed the genuine im- portation of Messrs. A. Gibbs and Sons, ^g lOs per ton, or ^Q 5s in quantities of five tons or upwards. EDWARD PURSE Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/the-cottage-gardener-gardening-gardening-the-cottage-gakdeneradvertisements-rrile-london-manure-com-j-panv-beg-to-offer-jis-under-corn-manure-most-valuable-for-spring-drees-ing-concentrated-urate-super-phosphate-of-lime-nitrate-of-soda-sulphate-of-ammonia-fishery-and-agricultural-salt-gypsum-fossil-bones-sulphuric-acid-and-every-other-artificial-manure-also-a-constant-supply-of-english-and-foreign-linseed-cake-peruvian-guano-guaranteed-the-genuine-im-portation-of-messrs-a-gibbs-and-sons-g-los-per-ton-or-q-5s-in-quantities-of-five-tons-or-upwards-edward-purse-image232499250.htmlRMRE774J–. The Cottage gardener. Gardening; Gardening. THE COTTAGE GAKDENER.—ADVERTISEMENTS. rrilE LONDON MANURE COM- J- PANV beg to offer Jis under:— Corn Manure, most valuable for spring drees- ing, Concentrated Urate, Super-Phosphate of Lime, Nitrate of Soda, Sulphate of Ammonia, Fishery and Agricultural Salt, Gypsum, Fossil Bones, Sulphuric Acid, and every other artificial manure ; also, a constant supply of English and Foreign Linseed Cake, Peruvian Guano, guaranteed the genuine im- portation of Messrs. A. Gibbs and Sons, ^g lOs per ton, or ^Q 5s in quantities of five tons or upwards. EDWARD PURSE
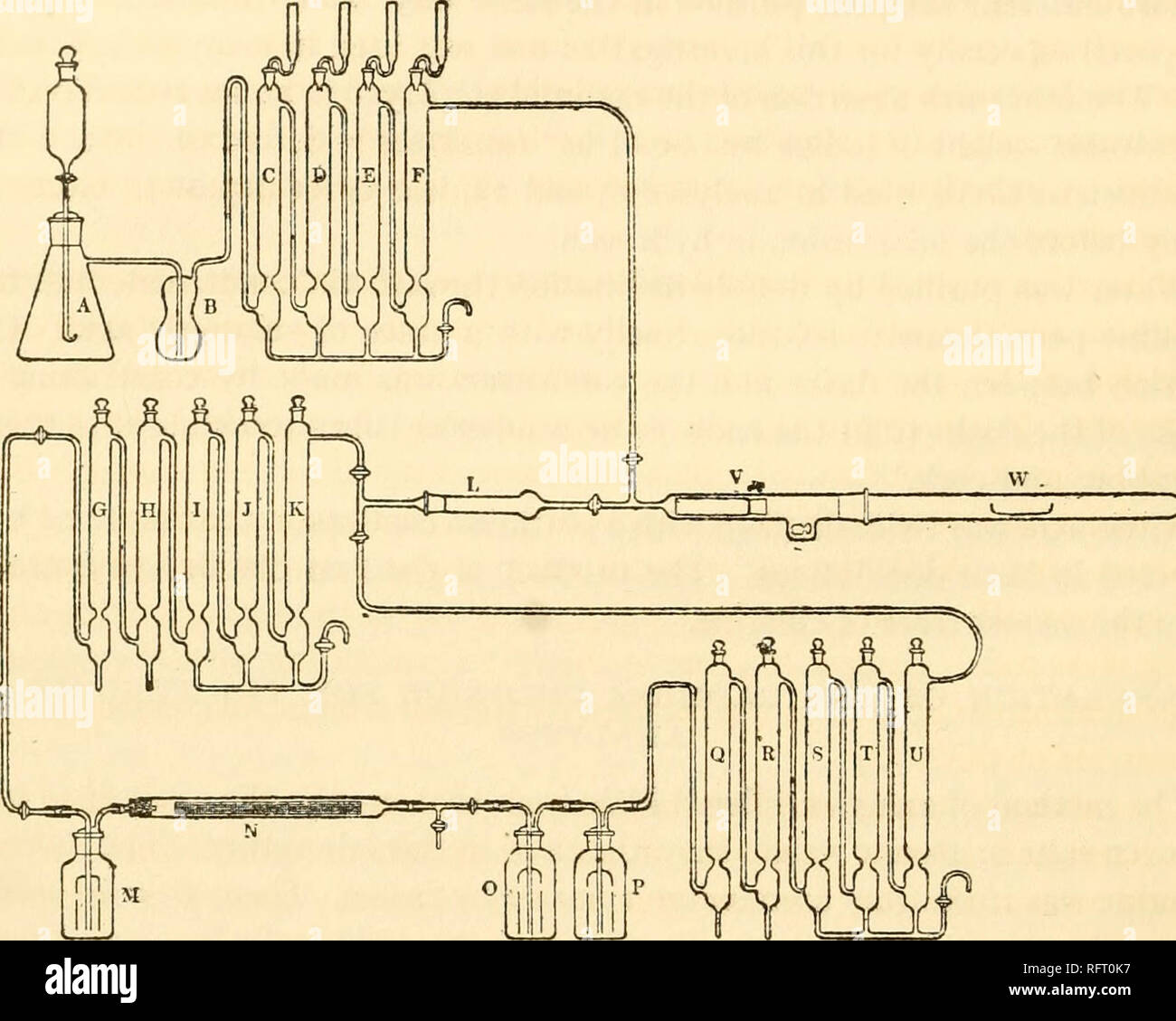 . Carnegie Institution of Washington publication. 8 RESEARCHES UPON ATOMIC WEIGHTS. gas was further dried by passing through a tube containing resublimed phos- phorus pentoxide. This tube is not shown in the diagram since it was elim- inated in the final series of experiments. Nitrogen was prepared by Wanklyn's method of passing air through concen- trated ammonia solution in the bottle M and then over hot copper gauze in the hard glass tube N. The excess of ammonia was removed by dilute sulphuric c*=<. Fig. I. — Apparatus for the fusion of chlorides in a current of hydrochloric-acid gas. ac Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/carnegie-institution-of-washington-publication-8-researches-upon-atomic-weights-gas-was-further-dried-by-passing-through-a-tube-containing-resublimed-phos-phorus-pentoxide-this-tube-is-not-shown-in-the-diagram-since-it-was-elim-inated-in-the-final-series-of-experiments-nitrogen-was-prepared-by-wanklyns-method-of-passing-air-through-concen-trated-ammonia-solution-in-the-bottle-m-and-then-over-hot-copper-gauze-in-the-hard-glass-tube-n-the-excess-of-ammonia-was-removed-by-dilute-sulphuric-c=lt-fig-i-apparatus-for-the-fusion-of-chlorides-in-a-current-of-hydrochloric-acid-gas-ac-image233482011.html
. Carnegie Institution of Washington publication. 8 RESEARCHES UPON ATOMIC WEIGHTS. gas was further dried by passing through a tube containing resublimed phos- phorus pentoxide. This tube is not shown in the diagram since it was elim- inated in the final series of experiments. Nitrogen was prepared by Wanklyn's method of passing air through concen- trated ammonia solution in the bottle M and then over hot copper gauze in the hard glass tube N. The excess of ammonia was removed by dilute sulphuric c*=<. Fig. I. — Apparatus for the fusion of chlorides in a current of hydrochloric-acid gas. ac Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/carnegie-institution-of-washington-publication-8-researches-upon-atomic-weights-gas-was-further-dried-by-passing-through-a-tube-containing-resublimed-phos-phorus-pentoxide-this-tube-is-not-shown-in-the-diagram-since-it-was-elim-inated-in-the-final-series-of-experiments-nitrogen-was-prepared-by-wanklyns-method-of-passing-air-through-concen-trated-ammonia-solution-in-the-bottle-m-and-then-over-hot-copper-gauze-in-the-hard-glass-tube-n-the-excess-of-ammonia-was-removed-by-dilute-sulphuric-c=lt-fig-i-apparatus-for-the-fusion-of-chlorides-in-a-current-of-hydrochloric-acid-gas-ac-image233482011.htmlRMRFT0K7–. Carnegie Institution of Washington publication. 8 RESEARCHES UPON ATOMIC WEIGHTS. gas was further dried by passing through a tube containing resublimed phos- phorus pentoxide. This tube is not shown in the diagram since it was elim- inated in the final series of experiments. Nitrogen was prepared by Wanklyn's method of passing air through concen- trated ammonia solution in the bottle M and then over hot copper gauze in the hard glass tube N. The excess of ammonia was removed by dilute sulphuric c*=<. Fig. I. — Apparatus for the fusion of chlorides in a current of hydrochloric-acid gas. ac
 . British bee journal & bee-keepers adviser. Bees. 190 THE BEITISH BEE JOURNAL. [June 15, 191G. then holding the test tube slantwise pour some strong sulphuric acid down the side of the tube. A brown ring will appear if nitrates or nitrites are present. Another method of detecting ammonia is to heat a weighed mixture of soil and soda-lime, and lead away by means of a tube the am- moniacal vapour given off. If hydro- chloric acid gas is then introduced, dense white fumes of ammonium chloride will be observed. For a complete quantitative estimation of nitrogen costty apparatus and considerab Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/british-bee-journal-amp-bee-keepers-adviser-bees-190-the-beitish-bee-journal-june-15-191g-then-holding-the-test-tube-slantwise-pour-some-strong-sulphuric-acid-down-the-side-of-the-tube-a-brown-ring-will-appear-if-nitrates-or-nitrites-are-present-another-method-of-detecting-ammonia-is-to-heat-a-weighed-mixture-of-soil-and-soda-lime-and-lead-away-by-means-of-a-tube-the-am-moniacal-vapour-given-off-if-hydro-chloric-acid-gas-is-then-introduced-dense-white-fumes-of-ammonium-chloride-will-be-observed-for-a-complete-quantitative-estimation-of-nitrogen-costty-apparatus-and-considerab-image234240597.html
. British bee journal & bee-keepers adviser. Bees. 190 THE BEITISH BEE JOURNAL. [June 15, 191G. then holding the test tube slantwise pour some strong sulphuric acid down the side of the tube. A brown ring will appear if nitrates or nitrites are present. Another method of detecting ammonia is to heat a weighed mixture of soil and soda-lime, and lead away by means of a tube the am- moniacal vapour given off. If hydro- chloric acid gas is then introduced, dense white fumes of ammonium chloride will be observed. For a complete quantitative estimation of nitrogen costty apparatus and considerab Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/british-bee-journal-amp-bee-keepers-adviser-bees-190-the-beitish-bee-journal-june-15-191g-then-holding-the-test-tube-slantwise-pour-some-strong-sulphuric-acid-down-the-side-of-the-tube-a-brown-ring-will-appear-if-nitrates-or-nitrites-are-present-another-method-of-detecting-ammonia-is-to-heat-a-weighed-mixture-of-soil-and-soda-lime-and-lead-away-by-means-of-a-tube-the-am-moniacal-vapour-given-off-if-hydro-chloric-acid-gas-is-then-introduced-dense-white-fumes-of-ammonium-chloride-will-be-observed-for-a-complete-quantitative-estimation-of-nitrogen-costty-apparatus-and-considerab-image234240597.htmlRMRH2G7H–. British bee journal & bee-keepers adviser. Bees. 190 THE BEITISH BEE JOURNAL. [June 15, 191G. then holding the test tube slantwise pour some strong sulphuric acid down the side of the tube. A brown ring will appear if nitrates or nitrites are present. Another method of detecting ammonia is to heat a weighed mixture of soil and soda-lime, and lead away by means of a tube the am- moniacal vapour given off. If hydro- chloric acid gas is then introduced, dense white fumes of ammonium chloride will be observed. For a complete quantitative estimation of nitrogen costty apparatus and considerab
 . Elements of agricultural chemistry and geology. Agricultural chemistry. 34 SULPHURIC AND PHOSPHORIC ACIDS. This acid combines with potash, soda, lime, magnesia, and ammonia, and forms sulphates. These sulphates exist in the soil, and when dissolved by water are conveyed into the sap of plants, and supply the sulphur which is necessary for the formation of their growing parts. The strong acid is now employed largely for dissolving bones and the fossil phosphates of lime, from which the artificial ma- nure known as super-phosphate of lime is manufactured. In a diluted state it has been employe Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/elements-of-agricultural-chemistry-and-geology-agricultural-chemistry-34-sulphuric-and-phosphoric-acids-this-acid-combines-with-potash-soda-lime-magnesia-and-ammonia-and-forms-sulphates-these-sulphates-exist-in-the-soil-and-when-dissolved-by-water-are-conveyed-into-the-sap-of-plants-and-supply-the-sulphur-which-is-necessary-for-the-formation-of-their-growing-parts-the-strong-acid-is-now-employed-largely-for-dissolving-bones-and-the-fossil-phosphates-of-lime-from-which-the-artificial-ma-nure-known-as-super-phosphate-of-lime-is-manufactured-in-a-diluted-state-it-has-been-employe-image231667428.html
. Elements of agricultural chemistry and geology. Agricultural chemistry. 34 SULPHURIC AND PHOSPHORIC ACIDS. This acid combines with potash, soda, lime, magnesia, and ammonia, and forms sulphates. These sulphates exist in the soil, and when dissolved by water are conveyed into the sap of plants, and supply the sulphur which is necessary for the formation of their growing parts. The strong acid is now employed largely for dissolving bones and the fossil phosphates of lime, from which the artificial ma- nure known as super-phosphate of lime is manufactured. In a diluted state it has been employe Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/elements-of-agricultural-chemistry-and-geology-agricultural-chemistry-34-sulphuric-and-phosphoric-acids-this-acid-combines-with-potash-soda-lime-magnesia-and-ammonia-and-forms-sulphates-these-sulphates-exist-in-the-soil-and-when-dissolved-by-water-are-conveyed-into-the-sap-of-plants-and-supply-the-sulphur-which-is-necessary-for-the-formation-of-their-growing-parts-the-strong-acid-is-now-employed-largely-for-dissolving-bones-and-the-fossil-phosphates-of-lime-from-which-the-artificial-ma-nure-known-as-super-phosphate-of-lime-is-manufactured-in-a-diluted-state-it-has-been-employe-image231667428.htmlRMRCWA4M–. Elements of agricultural chemistry and geology. Agricultural chemistry. 34 SULPHURIC AND PHOSPHORIC ACIDS. This acid combines with potash, soda, lime, magnesia, and ammonia, and forms sulphates. These sulphates exist in the soil, and when dissolved by water are conveyed into the sap of plants, and supply the sulphur which is necessary for the formation of their growing parts. The strong acid is now employed largely for dissolving bones and the fossil phosphates of lime, from which the artificial ma- nure known as super-phosphate of lime is manufactured. In a diluted state it has been employe
 . Elements of agricultural chemistry and geology. Agricultural chemistry. 18 PROPERTIES OF CARBONIC ACID. greater part of the oxygen and nitrogen also, enter into plants in a state of chemical combination with other substances. The carbon is taken up chiefly in the state of carbonic acid, and of certain other soluble compounds which exist in the soil; the hydrogen and oxygen in the form of water-; the nitrogen chiefly, it is supposed, in those of ammonia, of certain other soluble sub- stances containing nitrogen, and of nitric acid; and the sulphur and phosphorus in those of sulphuric and phos Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/elements-of-agricultural-chemistry-and-geology-agricultural-chemistry-18-properties-of-carbonic-acid-greater-part-of-the-oxygen-and-nitrogen-also-enter-into-plants-in-a-state-of-chemical-combination-with-other-substances-the-carbon-is-taken-up-chiefly-in-the-state-of-carbonic-acid-and-of-certain-other-soluble-compounds-which-exist-in-the-soil-the-hydrogen-and-oxygen-in-the-form-of-water-the-nitrogen-chiefly-it-is-supposed-in-those-of-ammonia-of-certain-other-soluble-sub-stances-containing-nitrogen-and-of-nitric-acid-and-the-sulphur-and-phosphorus-in-those-of-sulphuric-and-phos-image231667435.html
. Elements of agricultural chemistry and geology. Agricultural chemistry. 18 PROPERTIES OF CARBONIC ACID. greater part of the oxygen and nitrogen also, enter into plants in a state of chemical combination with other substances. The carbon is taken up chiefly in the state of carbonic acid, and of certain other soluble compounds which exist in the soil; the hydrogen and oxygen in the form of water-; the nitrogen chiefly, it is supposed, in those of ammonia, of certain other soluble sub- stances containing nitrogen, and of nitric acid; and the sulphur and phosphorus in those of sulphuric and phos Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/elements-of-agricultural-chemistry-and-geology-agricultural-chemistry-18-properties-of-carbonic-acid-greater-part-of-the-oxygen-and-nitrogen-also-enter-into-plants-in-a-state-of-chemical-combination-with-other-substances-the-carbon-is-taken-up-chiefly-in-the-state-of-carbonic-acid-and-of-certain-other-soluble-compounds-which-exist-in-the-soil-the-hydrogen-and-oxygen-in-the-form-of-water-the-nitrogen-chiefly-it-is-supposed-in-those-of-ammonia-of-certain-other-soluble-sub-stances-containing-nitrogen-and-of-nitric-acid-and-the-sulphur-and-phosphorus-in-those-of-sulphuric-and-phos-image231667435.htmlRMRCWA4Y–. Elements of agricultural chemistry and geology. Agricultural chemistry. 18 PROPERTIES OF CARBONIC ACID. greater part of the oxygen and nitrogen also, enter into plants in a state of chemical combination with other substances. The carbon is taken up chiefly in the state of carbonic acid, and of certain other soluble compounds which exist in the soil; the hydrogen and oxygen in the form of water-; the nitrogen chiefly, it is supposed, in those of ammonia, of certain other soluble sub- stances containing nitrogen, and of nitric acid; and the sulphur and phosphorus in those of sulphuric and phos