Quick filters:
Camphoric acid Stock Photos and Images
 Derivatives of isocamphoric acid, decomposition of isodihydroaminocampholytic acid with nitrous acid . proposed in 1895, based upon the fact that camphor oxime loses waterto form campholenic nitrile . (f) Bouveaults second formula,1 proposed in 1897 . The occurrence of trimethyl succinic acid amongthe oxidation products of camphor, and the proof offered by Walkerfand Noyes^ that the two carboxyl groups of camphoric acid are onadjacent carbon atoms led Eouveault to propose this formula. Owing to the ease with which the camphor molecule rearrangesit was almost impossible to decide between these Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/derivatives-of-isocamphoric-acid-decomposition-of-isodihydroaminocampholytic-acid-with-nitrous-acid-proposed-in-1895-based-upon-the-fact-that-camphor-oxime-loses-waterto-form-campholenic-nitrile-f-bouveaults-second-formula1-proposed-in-1897-the-occurrence-of-trimethyl-succinic-acid-amongthe-oxidation-products-of-camphor-and-the-proof-offered-by-walkerfand-noyes-that-the-two-carboxyl-groups-of-camphoric-acid-are-onadjacent-carbon-atoms-led-eouveault-to-propose-this-formula-owing-to-the-ease-with-which-the-camphor-molecule-rearrangesit-was-almost-impossible-to-decide-between-these-image340129348.html
Derivatives of isocamphoric acid, decomposition of isodihydroaminocampholytic acid with nitrous acid . proposed in 1895, based upon the fact that camphor oxime loses waterto form campholenic nitrile . (f) Bouveaults second formula,1 proposed in 1897 . The occurrence of trimethyl succinic acid amongthe oxidation products of camphor, and the proof offered by Walkerfand Noyes^ that the two carboxyl groups of camphoric acid are onadjacent carbon atoms led Eouveault to propose this formula. Owing to the ease with which the camphor molecule rearrangesit was almost impossible to decide between these Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/derivatives-of-isocamphoric-acid-decomposition-of-isodihydroaminocampholytic-acid-with-nitrous-acid-proposed-in-1895-based-upon-the-fact-that-camphor-oxime-loses-waterto-form-campholenic-nitrile-f-bouveaults-second-formula1-proposed-in-1897-the-occurrence-of-trimethyl-succinic-acid-amongthe-oxidation-products-of-camphor-and-the-proof-offered-by-walkerfand-noyes-that-the-two-carboxyl-groups-of-camphoric-acid-are-onadjacent-carbon-atoms-led-eouveault-to-propose-this-formula-owing-to-the-ease-with-which-the-camphor-molecule-rearrangesit-was-almost-impossible-to-decide-between-these-image340129348.htmlRM2ANA6CM–Derivatives of isocamphoric acid, decomposition of isodihydroaminocampholytic acid with nitrous acid . proposed in 1895, based upon the fact that camphor oxime loses waterto form campholenic nitrile . (f) Bouveaults second formula,1 proposed in 1897 . The occurrence of trimethyl succinic acid amongthe oxidation products of camphor, and the proof offered by Walkerfand Noyes^ that the two carboxyl groups of camphoric acid are onadjacent carbon atoms led Eouveault to propose this formula. Owing to the ease with which the camphor molecule rearrangesit was almost impossible to decide between these
 An old printed photograph showing natives harvesting camphor in the camphor forests of Formosa a short-lived republic that existed on the island of Taiwan between May and October 1895 when the capital Taipei / Tainan was captured by the Japanese.Camphor is a waxy, flammable, transparent substance with a strong smell extracted from the wood of the camphor laurel (Cinnamomum camphora) and other members of the plant kingdom. It is used in the manufacture of moth balls. Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/an-old-printed-photograph-showing-natives-harvesting-camphor-in-the-camphor-forests-of-formosa-a-short-lived-republic-that-existed-on-the-island-of-taiwan-between-may-and-october-1895-when-the-capital-taipei-tainan-was-captured-by-the-japanesecamphor-is-a-waxy-flammable-transparent-substance-with-a-strong-smell-extracted-from-the-wood-of-the-camphor-laurel-cinnamomum-camphora-and-other-members-of-the-plant-kingdom-it-is-used-in-the-manufacture-of-moth-balls-image366454008.html
An old printed photograph showing natives harvesting camphor in the camphor forests of Formosa a short-lived republic that existed on the island of Taiwan between May and October 1895 when the capital Taipei / Tainan was captured by the Japanese.Camphor is a waxy, flammable, transparent substance with a strong smell extracted from the wood of the camphor laurel (Cinnamomum camphora) and other members of the plant kingdom. It is used in the manufacture of moth balls. Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/an-old-printed-photograph-showing-natives-harvesting-camphor-in-the-camphor-forests-of-formosa-a-short-lived-republic-that-existed-on-the-island-of-taiwan-between-may-and-october-1895-when-the-capital-taipei-tainan-was-captured-by-the-japanesecamphor-is-a-waxy-flammable-transparent-substance-with-a-strong-smell-extracted-from-the-wood-of-the-camphor-laurel-cinnamomum-camphora-and-other-members-of-the-plant-kingdom-it-is-used-in-the-manufacture-of-moth-balls-image366454008.htmlRM2C85BR4–An old printed photograph showing natives harvesting camphor in the camphor forests of Formosa a short-lived republic that existed on the island of Taiwan between May and October 1895 when the capital Taipei / Tainan was captured by the Japanese.Camphor is a waxy, flammable, transparent substance with a strong smell extracted from the wood of the camphor laurel (Cinnamomum camphora) and other members of the plant kingdom. It is used in the manufacture of moth balls.
 Nicolas Louis Vauquelin, French Pharmacist and Chemist Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/stock-photo-nicolas-louis-vauquelin-french-pharmacist-and-chemist-135088953.html
Nicolas Louis Vauquelin, French Pharmacist and Chemist Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/stock-photo-nicolas-louis-vauquelin-french-pharmacist-and-chemist-135088953.htmlRMHRNR9D–Nicolas Louis Vauquelin, French Pharmacist and Chemist
 Derivatives of isocamphoric acid, decomposition of isodihydroaminocampholytic acid with nitrous acid . 113, 825; 2. Jr. Am. Oh. Soc. 32, 1671. Berichte 25.12?, 107. 3. Jr. Am. Ch. Soo. 35, 77 . 5. Jr. Ch. Soc. 77, 387 . 21 Chlorides of the Camphoric Acids CgHl4 (C0C1)2 The di-chloride of d-camphoric acid was first prepared by1 Moitessier (1861) by mixing camphoric acid and phosphorus penta- chloride. The product decomposed on heating and was not obtained2 pure. Marsh (1889), however, distilled it under re duced pressure . b. p. = 140 /15 mm. A product free from chloro-camphoryl chloride3 by Ha Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/derivatives-of-isocamphoric-acid-decomposition-of-isodihydroaminocampholytic-acid-with-nitrous-acid-113-825-2-jr-am-oh-soc-32-1671-berichte-2512-107-3-jr-am-ch-soo-35-77-5-jr-ch-soc-77-387-21-chlorides-of-the-camphoric-acids-cghl4-c0c12-the-di-chloride-of-d-camphoric-acid-was-first-prepared-by1-moitessier-1861-by-mixing-camphoric-acid-and-phosphorus-penta-chloride-the-product-decomposed-on-heating-and-was-not-obtained2-pure-marsh-1889-however-distilled-it-under-re-duced-pressure-b-p-=-140-15-mm-a-product-free-from-chloro-camphoryl-chloride3-by-ha-image340128540.html
Derivatives of isocamphoric acid, decomposition of isodihydroaminocampholytic acid with nitrous acid . 113, 825; 2. Jr. Am. Oh. Soc. 32, 1671. Berichte 25.12?, 107. 3. Jr. Am. Ch. Soo. 35, 77 . 5. Jr. Ch. Soc. 77, 387 . 21 Chlorides of the Camphoric Acids CgHl4 (C0C1)2 The di-chloride of d-camphoric acid was first prepared by1 Moitessier (1861) by mixing camphoric acid and phosphorus penta- chloride. The product decomposed on heating and was not obtained2 pure. Marsh (1889), however, distilled it under re duced pressure . b. p. = 140 /15 mm. A product free from chloro-camphoryl chloride3 by Ha Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/derivatives-of-isocamphoric-acid-decomposition-of-isodihydroaminocampholytic-acid-with-nitrous-acid-113-825-2-jr-am-oh-soc-32-1671-berichte-2512-107-3-jr-am-ch-soo-35-77-5-jr-ch-soc-77-387-21-chlorides-of-the-camphoric-acids-cghl4-c0c12-the-di-chloride-of-d-camphoric-acid-was-first-prepared-by1-moitessier-1861-by-mixing-camphoric-acid-and-phosphorus-penta-chloride-the-product-decomposed-on-heating-and-was-not-obtained2-pure-marsh-1889-however-distilled-it-under-re-duced-pressure-b-p-=-140-15-mm-a-product-free-from-chloro-camphoryl-chloride3-by-ha-image340128540.htmlRM2ANA5BT–Derivatives of isocamphoric acid, decomposition of isodihydroaminocampholytic acid with nitrous acid . 113, 825; 2. Jr. Am. Oh. Soc. 32, 1671. Berichte 25.12?, 107. 3. Jr. Am. Ch. Soo. 35, 77 . 5. Jr. Ch. Soc. 77, 387 . 21 Chlorides of the Camphoric Acids CgHl4 (C0C1)2 The di-chloride of d-camphoric acid was first prepared by1 Moitessier (1861) by mixing camphoric acid and phosphorus penta- chloride. The product decomposed on heating and was not obtained2 pure. Marsh (1889), however, distilled it under re duced pressure . b. p. = 140 /15 mm. A product free from chloro-camphoryl chloride3 by Ha
 Nicolas Louis Vauquelin, French Pharmacist and Chemist Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/stock-photo-nicolas-louis-vauquelin-french-pharmacist-and-chemist-135088954.html
Nicolas Louis Vauquelin, French Pharmacist and Chemist Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/stock-photo-nicolas-louis-vauquelin-french-pharmacist-and-chemist-135088954.htmlRMHRNR9E–Nicolas Louis Vauquelin, French Pharmacist and Chemist
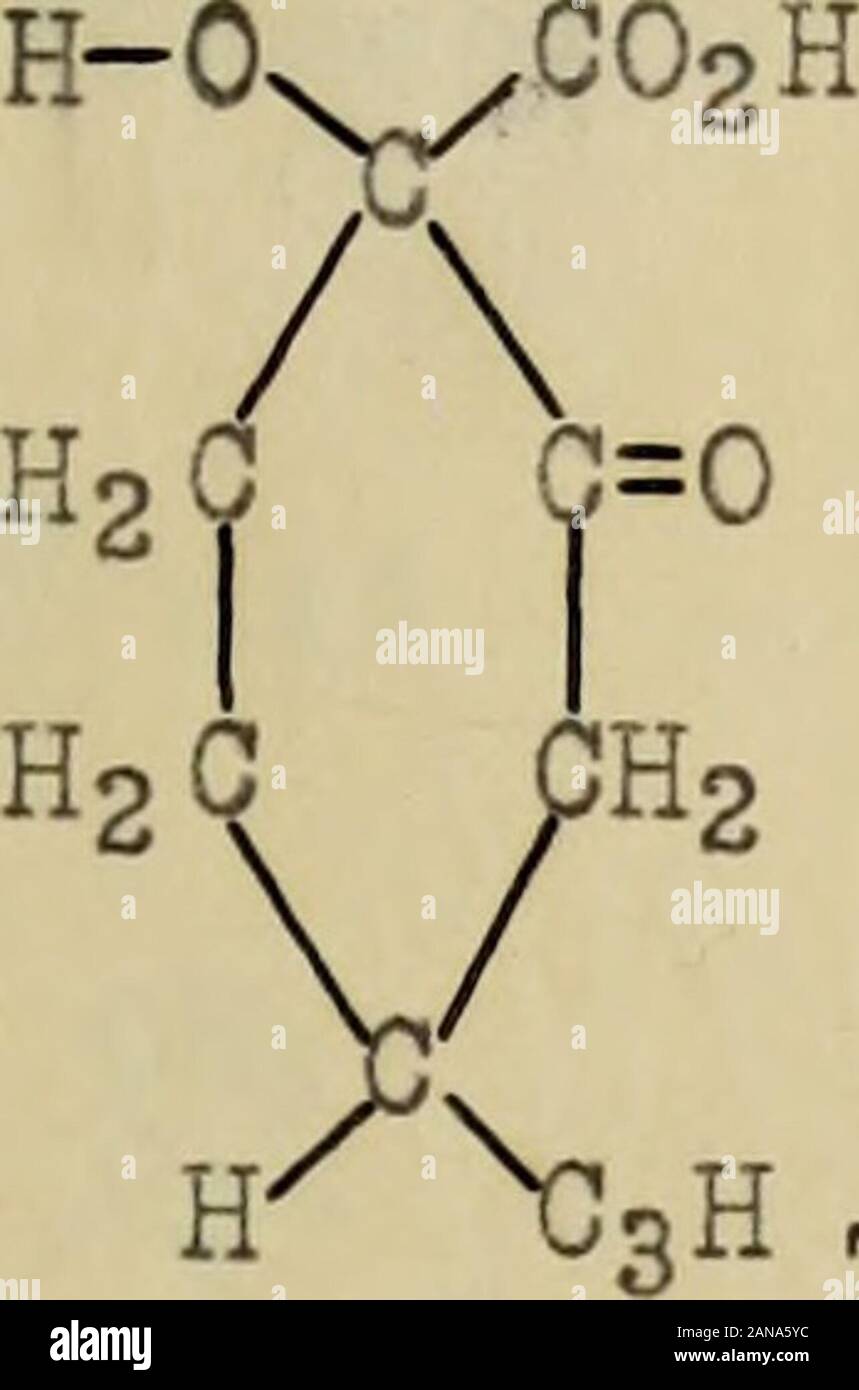 Derivatives of isocamphoric acid, decomposition of isodihydroaminocampholytic acid with nitrous acid . tertiary acid.W. Weyl^ (1868) suggested that camphoric acid was either a normal ^COOH dibasic acid, CsHl4 > or an hydroxylated acetone acid, ^COOH 1. 0. Aschan, Ann. 316, 209. 2. Roscoe and Schorlemmer, Treatise on Chemistry (1889) vol. Ill,pt . 5, p . 412 . 3. Ann .22, 38, 50 . 4. J. W. Bruhl, Eerichte 24, 3409. 5. Berichte 1, 94. X0H (GqIi40) , in which the hydroxy 1 group functioned, as an acid. v ^00 OH V. Meyer^ (1870) expressed the opinion that it was the normal di-basic acid. N. Men Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/derivatives-of-isocamphoric-acid-decomposition-of-isodihydroaminocampholytic-acid-with-nitrous-acid-tertiary-acidw-weyl-1868-suggested-that-camphoric-acid-was-either-a-normal-cooh-dibasic-acid-cshl4-gt-or-an-hydroxylated-acetone-acid-cooh-1-0-aschan-ann-316-209-2-roscoe-and-schorlemmer-treatise-on-chemistry-1889-vol-illpt-5-p-412-3-ann-22-38-50-4-j-w-bruhl-eerichte-24-3409-5-berichte-1-94-x0h-gqii40-in-which-the-hydroxy-1-group-functioned-as-an-acid-v-00-oh-v-meyer-1870-expressed-the-opinion-that-it-was-the-normal-di-basic-acid-n-men-image340128976.html
Derivatives of isocamphoric acid, decomposition of isodihydroaminocampholytic acid with nitrous acid . tertiary acid.W. Weyl^ (1868) suggested that camphoric acid was either a normal ^COOH dibasic acid, CsHl4 > or an hydroxylated acetone acid, ^COOH 1. 0. Aschan, Ann. 316, 209. 2. Roscoe and Schorlemmer, Treatise on Chemistry (1889) vol. Ill,pt . 5, p . 412 . 3. Ann .22, 38, 50 . 4. J. W. Bruhl, Eerichte 24, 3409. 5. Berichte 1, 94. X0H (GqIi40) , in which the hydroxy 1 group functioned, as an acid. v ^00 OH V. Meyer^ (1870) expressed the opinion that it was the normal di-basic acid. N. Men Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/derivatives-of-isocamphoric-acid-decomposition-of-isodihydroaminocampholytic-acid-with-nitrous-acid-tertiary-acidw-weyl-1868-suggested-that-camphoric-acid-was-either-a-normal-cooh-dibasic-acid-cshl4-gt-or-an-hydroxylated-acetone-acid-cooh-1-0-aschan-ann-316-209-2-roscoe-and-schorlemmer-treatise-on-chemistry-1889-vol-illpt-5-p-412-3-ann-22-38-50-4-j-w-bruhl-eerichte-24-3409-5-berichte-1-94-x0h-gqii40-in-which-the-hydroxy-1-group-functioned-as-an-acid-v-00-oh-v-meyer-1870-expressed-the-opinion-that-it-was-the-normal-di-basic-acid-n-men-image340128976.htmlRM2ANA5YC–Derivatives of isocamphoric acid, decomposition of isodihydroaminocampholytic acid with nitrous acid . tertiary acid.W. Weyl^ (1868) suggested that camphoric acid was either a normal ^COOH dibasic acid, CsHl4 > or an hydroxylated acetone acid, ^COOH 1. 0. Aschan, Ann. 316, 209. 2. Roscoe and Schorlemmer, Treatise on Chemistry (1889) vol. Ill,pt . 5, p . 412 . 3. Ann .22, 38, 50 . 4. J. W. Bruhl, Eerichte 24, 3409. 5. Berichte 1, 94. X0H (GqIi40) , in which the hydroxy 1 group functioned, as an acid. v ^00 OH V. Meyer^ (1870) expressed the opinion that it was the normal di-basic acid. N. Men
 Nicolas Louis Vauquelin, French Pharmacist and Chemist Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/stock-photo-nicolas-louis-vauquelin-french-pharmacist-and-chemist-135088955.html
Nicolas Louis Vauquelin, French Pharmacist and Chemist Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/stock-photo-nicolas-louis-vauquelin-french-pharmacist-and-chemist-135088955.htmlRMHRNR9F–Nicolas Louis Vauquelin, French Pharmacist and Chemist
 . Annual report on essential oils, synthetic perfumes, &c. Essences and essential oils; Perfumes. Notes on scientific research. 99 lected in the cooler. After treating the distillate with 5 per cent, soda lye it was once more distilled over metallic sodium. The p-cymene obtained in this way had the following constants:—b. p. 176 to 176.5°; nD12.5o 1.4905. Since purified ^-cyrriene is an important solvent Wheeler determined the solubility of some compounds in this substance at different temperatures: 100 g. of ^-cymene dissolved 1.53 g. of ^-camphoric acid at 100°, 113.85 g. of thymol, 6.53 Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/annual-report-on-essential-oils-synthetic-perfumes-ampc-essences-and-essential-oils-perfumes-notes-on-scientific-research-99-lected-in-the-cooler-after-treating-the-distillate-with-5-per-cent-soda-lye-it-was-once-more-distilled-over-metallic-sodium-the-p-cymene-obtained-in-this-way-had-the-following-constantsb-p-176-to-1765-nd125o-14905-since-purified-cyrriene-is-an-important-solvent-wheeler-determined-the-solubility-of-some-compounds-in-this-substance-at-different-temperatures-100-g-of-cymene-dissolved-153-g-of-camphoric-acid-at-100-11385-g-of-thymol-653-image236200212.html
. Annual report on essential oils, synthetic perfumes, &c. Essences and essential oils; Perfumes. Notes on scientific research. 99 lected in the cooler. After treating the distillate with 5 per cent, soda lye it was once more distilled over metallic sodium. The p-cymene obtained in this way had the following constants:—b. p. 176 to 176.5°; nD12.5o 1.4905. Since purified ^-cyrriene is an important solvent Wheeler determined the solubility of some compounds in this substance at different temperatures: 100 g. of ^-cymene dissolved 1.53 g. of ^-camphoric acid at 100°, 113.85 g. of thymol, 6.53 Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/annual-report-on-essential-oils-synthetic-perfumes-ampc-essences-and-essential-oils-perfumes-notes-on-scientific-research-99-lected-in-the-cooler-after-treating-the-distillate-with-5-per-cent-soda-lye-it-was-once-more-distilled-over-metallic-sodium-the-p-cymene-obtained-in-this-way-had-the-following-constantsb-p-176-to-1765-nd125o-14905-since-purified-cyrriene-is-an-important-solvent-wheeler-determined-the-solubility-of-some-compounds-in-this-substance-at-different-temperatures-100-g-of-cymene-dissolved-153-g-of-camphoric-acid-at-100-11385-g-of-thymol-653-image236200212.htmlRMRM7RNT–. Annual report on essential oils, synthetic perfumes, &c. Essences and essential oils; Perfumes. Notes on scientific research. 99 lected in the cooler. After treating the distillate with 5 per cent, soda lye it was once more distilled over metallic sodium. The p-cymene obtained in this way had the following constants:—b. p. 176 to 176.5°; nD12.5o 1.4905. Since purified ^-cyrriene is an important solvent Wheeler determined the solubility of some compounds in this substance at different temperatures: 100 g. of ^-cymene dissolved 1.53 g. of ^-camphoric acid at 100°, 113.85 g. of thymol, 6.53
 Derivatives of isocamphoric acid, decomposition of isodihydroaminocampholytic acid with nitrous acid . o-camphoric anhydride. The evidence furnished by the formation ofd-campholytic acid as described above leaves no doubt on thispoint, however . The lactone proved to be a new compound. When pure it was awaxy solid melting at 114 -115° and having a specific rotation,( ) = -8.2°. These constants show it to be 1-campholytolactone The discrepancy of four degrees in the two melting points isnot so great as it would appear. Potter and Noyes obtained themelting point of 118-119° for d-campholytolacto Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/derivatives-of-isocamphoric-acid-decomposition-of-isodihydroaminocampholytic-acid-with-nitrous-acid-o-camphoric-anhydride-the-evidence-furnished-by-the-formation-ofd-campholytic-acid-as-described-above-leaves-no-doubt-on-thispoint-however-the-lactone-proved-to-be-a-new-compound-when-pure-it-was-awaxy-solid-melting-at-114-115-and-having-a-specific-rotation-=-82-these-constants-show-it-to-be-1-campholytolactone-the-discrepancy-of-four-degrees-in-the-two-melting-points-isnot-so-great-as-it-would-appear-potter-and-noyes-obtained-themelting-point-of-118-119-for-d-campholytolacto-image340124113.html
Derivatives of isocamphoric acid, decomposition of isodihydroaminocampholytic acid with nitrous acid . o-camphoric anhydride. The evidence furnished by the formation ofd-campholytic acid as described above leaves no doubt on thispoint, however . The lactone proved to be a new compound. When pure it was awaxy solid melting at 114 -115° and having a specific rotation,( ) = -8.2°. These constants show it to be 1-campholytolactone The discrepancy of four degrees in the two melting points isnot so great as it would appear. Potter and Noyes obtained themelting point of 118-119° for d-campholytolacto Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/derivatives-of-isocamphoric-acid-decomposition-of-isodihydroaminocampholytic-acid-with-nitrous-acid-o-camphoric-anhydride-the-evidence-furnished-by-the-formation-ofd-campholytic-acid-as-described-above-leaves-no-doubt-on-thispoint-however-the-lactone-proved-to-be-a-new-compound-when-pure-it-was-awaxy-solid-melting-at-114-115-and-having-a-specific-rotation-=-82-these-constants-show-it-to-be-1-campholytolactone-the-discrepancy-of-four-degrees-in-the-two-melting-points-isnot-so-great-as-it-would-appear-potter-and-noyes-obtained-themelting-point-of-118-119-for-d-campholytolacto-image340124113.htmlRM2AN9YNN–Derivatives of isocamphoric acid, decomposition of isodihydroaminocampholytic acid with nitrous acid . o-camphoric anhydride. The evidence furnished by the formation ofd-campholytic acid as described above leaves no doubt on thispoint, however . The lactone proved to be a new compound. When pure it was awaxy solid melting at 114 -115° and having a specific rotation,( ) = -8.2°. These constants show it to be 1-campholytolactone The discrepancy of four degrees in the two melting points isnot so great as it would appear. Potter and Noyes obtained themelting point of 118-119° for d-campholytolacto