Common salt cubes Stock Photos and Images
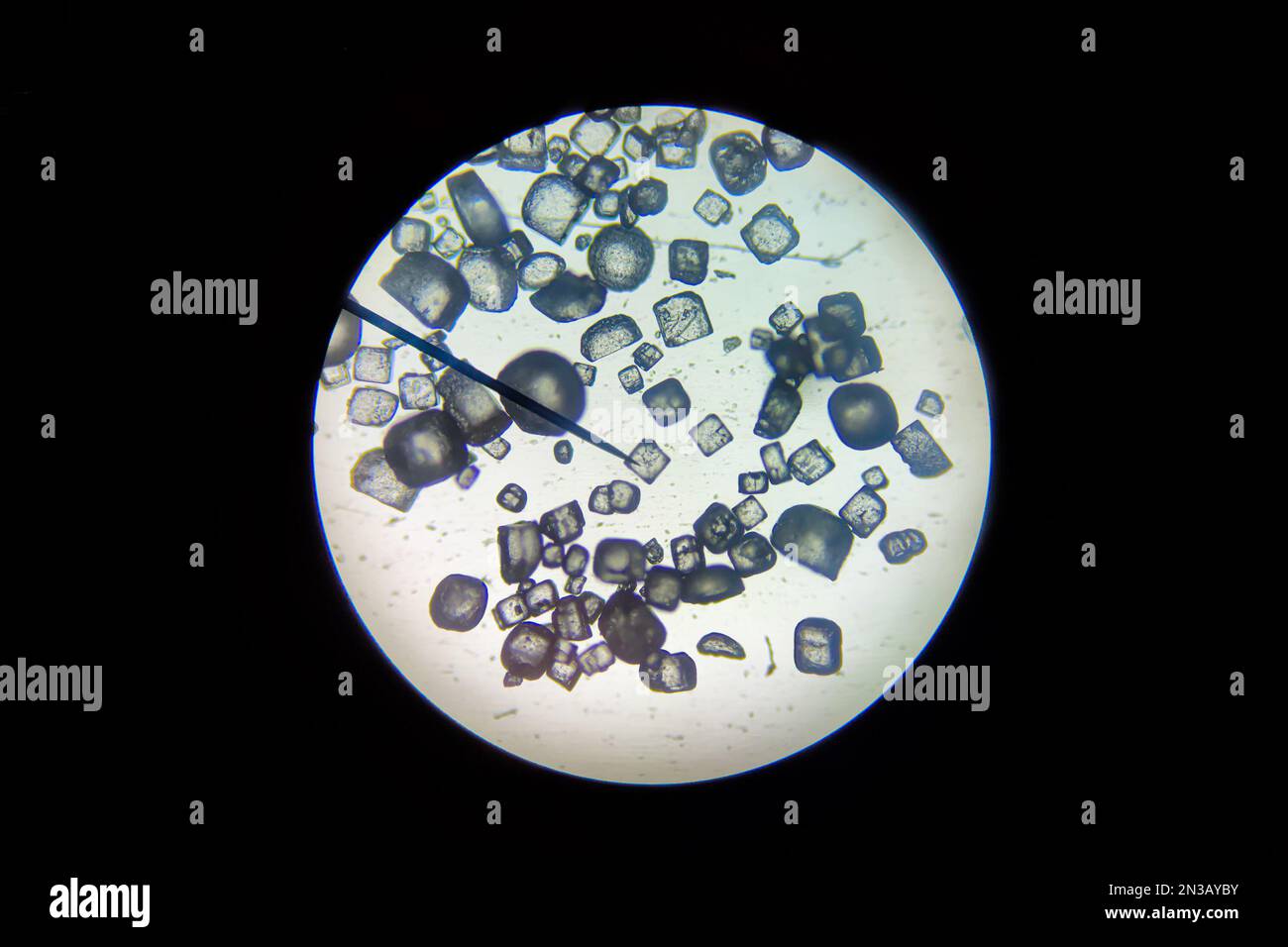 Close-up view of salt crystals (Sodium Chloryde) as seen through a microscope. Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/close-up-view-of-salt-crystals-sodium-chloryde-as-seen-through-a-microscope-image518396031.html
Close-up view of salt crystals (Sodium Chloryde) as seen through a microscope. Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/close-up-view-of-salt-crystals-sodium-chloryde-as-seen-through-a-microscope-image518396031.htmlRF2N3AYBY–Close-up view of salt crystals (Sodium Chloryde) as seen through a microscope.
 salt natural mineral cubes as very nice background Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/salt-natural-mineral-cubes-as-very-nice-background-image598570976.html
salt natural mineral cubes as very nice background Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/salt-natural-mineral-cubes-as-very-nice-background-image598570976.htmlRF2WNR7AT–salt natural mineral cubes as very nice background
 Picture of salt natural mineral cubes in the bowl with wooden scoop Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/picture-of-salt-natural-mineral-cubes-in-the-bowl-with-wooden-scoop-image388147340.html
Picture of salt natural mineral cubes in the bowl with wooden scoop Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/picture-of-salt-natural-mineral-cubes-in-the-bowl-with-wooden-scoop-image388147340.htmlRF2DFDHW0–Picture of salt natural mineral cubes in the bowl with wooden scoop
 Fish and seafood on ice cubes Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/fish-and-seafood-on-ice-cubes-image452084291.html
Fish and seafood on ice cubes Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/fish-and-seafood-on-ice-cubes-image452084291.htmlRF2H7E63F–Fish and seafood on ice cubes
 A text-book on chemistryFor the use of schools and colleges . s; under such circumstances, they aresaid to crystallize. Thus common salt will crystallizein cubes, and nitrate of potassa in six-sided prisms. The various geometrical forms which crystals can How is the hypothetical specific gravity of the vapor of carbondetermined ? Give the rule for calculating specific gravities of com-pound gases. SYSTEMS OF CRYSTALLIZATION. 205 thus assume may be divided into six classes or sys-tems: (1.) The Regular system. (2.) The Rhomhohedral system. (3.) The Square Prismatic system. (4.) The Right Prisma Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/a-text-book-on-chemistryfor-the-use-of-schools-and-colleges-s-under-such-circumstances-they-aresaid-to-crystallize-thus-common-salt-will-crystallizein-cubes-and-nitrate-of-potassa-in-six-sided-prisms-the-various-geometrical-forms-which-crystals-can-how-is-the-hypothetical-specific-gravity-of-the-vapor-of-carbondetermined-give-the-rule-for-calculating-specific-gravities-of-com-pound-gases-systems-of-crystallization-205-thus-assume-may-be-divided-into-six-classes-or-sys-tems-1-the-regular-system-2-the-rhomhohedral-system-3-the-square-prismatic-system-4-the-right-prisma-image343326477.html
A text-book on chemistryFor the use of schools and colleges . s; under such circumstances, they aresaid to crystallize. Thus common salt will crystallizein cubes, and nitrate of potassa in six-sided prisms. The various geometrical forms which crystals can How is the hypothetical specific gravity of the vapor of carbondetermined ? Give the rule for calculating specific gravities of com-pound gases. SYSTEMS OF CRYSTALLIZATION. 205 thus assume may be divided into six classes or sys-tems: (1.) The Regular system. (2.) The Rhomhohedral system. (3.) The Square Prismatic system. (4.) The Right Prisma Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/a-text-book-on-chemistryfor-the-use-of-schools-and-colleges-s-under-such-circumstances-they-aresaid-to-crystallize-thus-common-salt-will-crystallizein-cubes-and-nitrate-of-potassa-in-six-sided-prisms-the-various-geometrical-forms-which-crystals-can-how-is-the-hypothetical-specific-gravity-of-the-vapor-of-carbondetermined-give-the-rule-for-calculating-specific-gravities-of-com-pound-gases-systems-of-crystallization-205-thus-assume-may-be-divided-into-six-classes-or-sys-tems-1-the-regular-system-2-the-rhomhohedral-system-3-the-square-prismatic-system-4-the-right-prisma-image343326477.htmlRM2AXFTBW–A text-book on chemistryFor the use of schools and colleges . s; under such circumstances, they aresaid to crystallize. Thus common salt will crystallizein cubes, and nitrate of potassa in six-sided prisms. The various geometrical forms which crystals can How is the hypothetical specific gravity of the vapor of carbondetermined ? Give the rule for calculating specific gravities of com-pound gases. SYSTEMS OF CRYSTALLIZATION. 205 thus assume may be divided into six classes or sys-tems: (1.) The Regular system. (2.) The Rhomhohedral system. (3.) The Square Prismatic system. (4.) The Right Prisma
 . Elementary physics and chemistry: first stage . CRYSTALS AND CRYSTALLISATION—Continued. PRACTICAL WORK. Things required.—Crystals of washing-soda, sugar candy, borax, rock-crystal, blue vitriol, rock-salt, and alum. Flasks. Sand-bath. Laboratory burner. Tripod stand. Blotting paper. Magnify- ing glass. Evaporating basin. Sulphur. Iron spoon. Test-tubes. What to do. Evaporate a solu- tion of common salt by gently heating it, and, when the basin is dry, examine a little of the residue. Care- ful inspection will discover small cubes, the shape of some of which can be recognised by the unaided e Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/elementary-physics-and-chemistry-first-stage-crystals-and-crystallisationcontinued-practical-work-things-requiredcrystals-of-washing-soda-sugar-candy-borax-rock-crystal-blue-vitriol-rock-salt-and-alum-flasks-sand-bath-laboratory-burner-tripod-stand-blotting-paper-magnify-ing-glass-evaporating-basin-sulphur-iron-spoon-test-tubes-what-to-do-evaporate-a-solu-tion-of-common-salt-by-gently-heating-it-and-when-the-basin-is-dry-examine-a-little-of-the-residue-care-ful-inspection-will-discover-small-cubes-the-shape-of-some-of-which-can-be-recognised-by-the-unaided-e-image178403682.html
. Elementary physics and chemistry: first stage . CRYSTALS AND CRYSTALLISATION—Continued. PRACTICAL WORK. Things required.—Crystals of washing-soda, sugar candy, borax, rock-crystal, blue vitriol, rock-salt, and alum. Flasks. Sand-bath. Laboratory burner. Tripod stand. Blotting paper. Magnify- ing glass. Evaporating basin. Sulphur. Iron spoon. Test-tubes. What to do. Evaporate a solu- tion of common salt by gently heating it, and, when the basin is dry, examine a little of the residue. Care- ful inspection will discover small cubes, the shape of some of which can be recognised by the unaided e Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/elementary-physics-and-chemistry-first-stage-crystals-and-crystallisationcontinued-practical-work-things-requiredcrystals-of-washing-soda-sugar-candy-borax-rock-crystal-blue-vitriol-rock-salt-and-alum-flasks-sand-bath-laboratory-burner-tripod-stand-blotting-paper-magnify-ing-glass-evaporating-basin-sulphur-iron-spoon-test-tubes-what-to-do-evaporate-a-solu-tion-of-common-salt-by-gently-heating-it-and-when-the-basin-is-dry-examine-a-little-of-the-residue-care-ful-inspection-will-discover-small-cubes-the-shape-of-some-of-which-can-be-recognised-by-the-unaided-e-image178403682.htmlRMMA6YM2–. Elementary physics and chemistry: first stage . CRYSTALS AND CRYSTALLISATION—Continued. PRACTICAL WORK. Things required.—Crystals of washing-soda, sugar candy, borax, rock-crystal, blue vitriol, rock-salt, and alum. Flasks. Sand-bath. Laboratory burner. Tripod stand. Blotting paper. Magnify- ing glass. Evaporating basin. Sulphur. Iron spoon. Test-tubes. What to do. Evaporate a solu- tion of common salt by gently heating it, and, when the basin is dry, examine a little of the residue. Care- ful inspection will discover small cubes, the shape of some of which can be recognised by the unaided e
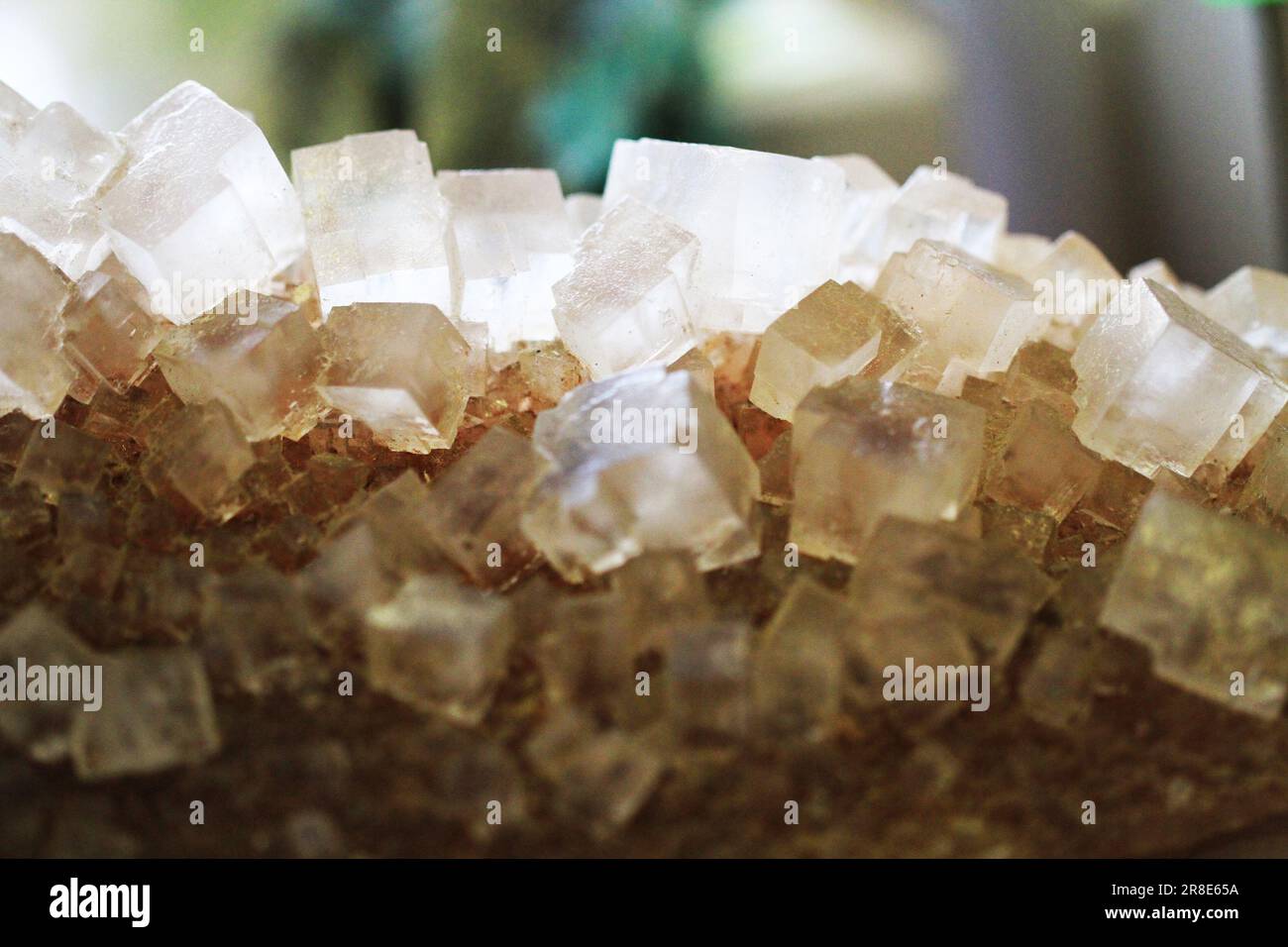 salt natural mineral cubes as very nice background Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/salt-natural-mineral-cubes-as-very-nice-background-image555961206.html
salt natural mineral cubes as very nice background Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/salt-natural-mineral-cubes-as-very-nice-background-image555961206.htmlRF2R8E65A–salt natural mineral cubes as very nice background
 . Elementary physics and chemistry: first stage. Science. CRYSTALS AND CRYSTALLISATION—Continued. PRACTICAL WORK. Things required.—Crystals of washing-soda, sugar candy, borax, rock-crystal, blue vitriol, rock-salt, and alum. Flasks. Sand-bath. Laboratory burner. Tripod stand. Blotting paper. Magnify- ing glass. Evaporating basin. Sulphur. Iron spoon. Test-tubes. What to do. Evaporate a solu- tion of common salt by gently heating it, and, when the basin is dry, examine a little of the residue. Care- ful inspection will discover small cubes, the shape of some of which can be recognised by the u Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/elementary-physics-and-chemistry-first-stage-science-crystals-and-crystallisationcontinued-practical-work-things-requiredcrystals-of-washing-soda-sugar-candy-borax-rock-crystal-blue-vitriol-rock-salt-and-alum-flasks-sand-bath-laboratory-burner-tripod-stand-blotting-paper-magnify-ing-glass-evaporating-basin-sulphur-iron-spoon-test-tubes-what-to-do-evaporate-a-solu-tion-of-common-salt-by-gently-heating-it-and-when-the-basin-is-dry-examine-a-little-of-the-residue-care-ful-inspection-will-discover-small-cubes-the-shape-of-some-of-which-can-be-recognised-by-the-u-image231775442.html
. Elementary physics and chemistry: first stage. Science. CRYSTALS AND CRYSTALLISATION—Continued. PRACTICAL WORK. Things required.—Crystals of washing-soda, sugar candy, borax, rock-crystal, blue vitriol, rock-salt, and alum. Flasks. Sand-bath. Laboratory burner. Tripod stand. Blotting paper. Magnify- ing glass. Evaporating basin. Sulphur. Iron spoon. Test-tubes. What to do. Evaporate a solu- tion of common salt by gently heating it, and, when the basin is dry, examine a little of the residue. Care- ful inspection will discover small cubes, the shape of some of which can be recognised by the u Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/elementary-physics-and-chemistry-first-stage-science-crystals-and-crystallisationcontinued-practical-work-things-requiredcrystals-of-washing-soda-sugar-candy-borax-rock-crystal-blue-vitriol-rock-salt-and-alum-flasks-sand-bath-laboratory-burner-tripod-stand-blotting-paper-magnify-ing-glass-evaporating-basin-sulphur-iron-spoon-test-tubes-what-to-do-evaporate-a-solu-tion-of-common-salt-by-gently-heating-it-and-when-the-basin-is-dry-examine-a-little-of-the-residue-care-ful-inspection-will-discover-small-cubes-the-shape-of-some-of-which-can-be-recognised-by-the-u-image231775442.htmlRMRD27XA–. Elementary physics and chemistry: first stage. Science. CRYSTALS AND CRYSTALLISATION—Continued. PRACTICAL WORK. Things required.—Crystals of washing-soda, sugar candy, borax, rock-crystal, blue vitriol, rock-salt, and alum. Flasks. Sand-bath. Laboratory burner. Tripod stand. Blotting paper. Magnify- ing glass. Evaporating basin. Sulphur. Iron spoon. Test-tubes. What to do. Evaporate a solu- tion of common salt by gently heating it, and, when the basin is dry, examine a little of the residue. Care- ful inspection will discover small cubes, the shape of some of which can be recognised by the u
 salt natural mineral cubes as very nice background Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/salt-natural-mineral-cubes-as-very-nice-background-image598570975.html
salt natural mineral cubes as very nice background Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/salt-natural-mineral-cubes-as-very-nice-background-image598570975.htmlRF2WNR7AR–salt natural mineral cubes as very nice background
 . Elementary physics and chemistry: first stage. Science. ELEMENTARY PHYSICS AND CHEMISTRY. LESSON XXXIII.. CRYSTALS AND CRYSTALLISATION—Continued. PRACTICAL WORK. Things required.—Crystals of washing-soda, sugar candy, borax, rock-crystal, blue vitriol, rock-salt, and alum. Flasks. Sand-bath. Laboratory burner. Tripod stand. Blotting paper. Magnify- ing glass. Evaporating basin. Sulphur. Iron spoon. Test-tubes. What to do. Evaporate a solu- tion of common salt by gently heating it, and, when the basin is dry, examine a little of the residue. Care- ful inspection will discover small cubes, the Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/elementary-physics-and-chemistry-first-stage-science-elementary-physics-and-chemistry-lesson-xxxiii-crystals-and-crystallisationcontinued-practical-work-things-requiredcrystals-of-washing-soda-sugar-candy-borax-rock-crystal-blue-vitriol-rock-salt-and-alum-flasks-sand-bath-laboratory-burner-tripod-stand-blotting-paper-magnify-ing-glass-evaporating-basin-sulphur-iron-spoon-test-tubes-what-to-do-evaporate-a-solu-tion-of-common-salt-by-gently-heating-it-and-when-the-basin-is-dry-examine-a-little-of-the-residue-care-ful-inspection-will-discover-small-cubes-the-image231775444.html
. Elementary physics and chemistry: first stage. Science. ELEMENTARY PHYSICS AND CHEMISTRY. LESSON XXXIII.. CRYSTALS AND CRYSTALLISATION—Continued. PRACTICAL WORK. Things required.—Crystals of washing-soda, sugar candy, borax, rock-crystal, blue vitriol, rock-salt, and alum. Flasks. Sand-bath. Laboratory burner. Tripod stand. Blotting paper. Magnify- ing glass. Evaporating basin. Sulphur. Iron spoon. Test-tubes. What to do. Evaporate a solu- tion of common salt by gently heating it, and, when the basin is dry, examine a little of the residue. Care- ful inspection will discover small cubes, the Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/elementary-physics-and-chemistry-first-stage-science-elementary-physics-and-chemistry-lesson-xxxiii-crystals-and-crystallisationcontinued-practical-work-things-requiredcrystals-of-washing-soda-sugar-candy-borax-rock-crystal-blue-vitriol-rock-salt-and-alum-flasks-sand-bath-laboratory-burner-tripod-stand-blotting-paper-magnify-ing-glass-evaporating-basin-sulphur-iron-spoon-test-tubes-what-to-do-evaporate-a-solu-tion-of-common-salt-by-gently-heating-it-and-when-the-basin-is-dry-examine-a-little-of-the-residue-care-ful-inspection-will-discover-small-cubes-the-image231775444.htmlRMRD27XC–. Elementary physics and chemistry: first stage. Science. ELEMENTARY PHYSICS AND CHEMISTRY. LESSON XXXIII.. CRYSTALS AND CRYSTALLISATION—Continued. PRACTICAL WORK. Things required.—Crystals of washing-soda, sugar candy, borax, rock-crystal, blue vitriol, rock-salt, and alum. Flasks. Sand-bath. Laboratory burner. Tripod stand. Blotting paper. Magnify- ing glass. Evaporating basin. Sulphur. Iron spoon. Test-tubes. What to do. Evaporate a solu- tion of common salt by gently heating it, and, when the basin is dry, examine a little of the residue. Care- ful inspection will discover small cubes, the
