Quick filters:
Copper sulphate solution Stock Photos and Images
 iron nail being copper plated by displacing copper from copper sulphate solution Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/iron-nail-being-copper-plated-by-displacing-copper-from-copper-sulphate-image1418175.html
iron nail being copper plated by displacing copper from copper sulphate solution Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/iron-nail-being-copper-plated-by-displacing-copper-from-copper-sulphate-image1418175.htmlRMANA3C0–iron nail being copper plated by displacing copper from copper sulphate solution
 Copper Sulfate solution in a glass gas jar Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/stock-photo-copper-sulfate-solution-in-a-glass-gas-jar-15298527.html
Copper Sulfate solution in a glass gas jar Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/stock-photo-copper-sulfate-solution-in-a-glass-gas-jar-15298527.htmlRMAMMP0G–Copper Sulfate solution in a glass gas jar
 Petri dishes of copper sulphate solution isolated on white background Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/stock-image-petri-dishes-of-copper-sulphate-solution-isolated-on-white-background-162029335.html
Petri dishes of copper sulphate solution isolated on white background Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/stock-image-petri-dishes-of-copper-sulphate-solution-isolated-on-white-background-162029335.htmlRFKBH21Y–Petri dishes of copper sulphate solution isolated on white background
 Heating water and copper sulphate solution with bunsen burner, leaving blue copper sulphate crystals Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/stock-photo-heating-water-and-copper-sulphate-solution-with-bunsen-burner-leaving-95273861.html
Heating water and copper sulphate solution with bunsen burner, leaving blue copper sulphate crystals Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/stock-photo-heating-water-and-copper-sulphate-solution-with-bunsen-burner-leaving-95273861.htmlRMFF02NW–Heating water and copper sulphate solution with bunsen burner, leaving blue copper sulphate crystals
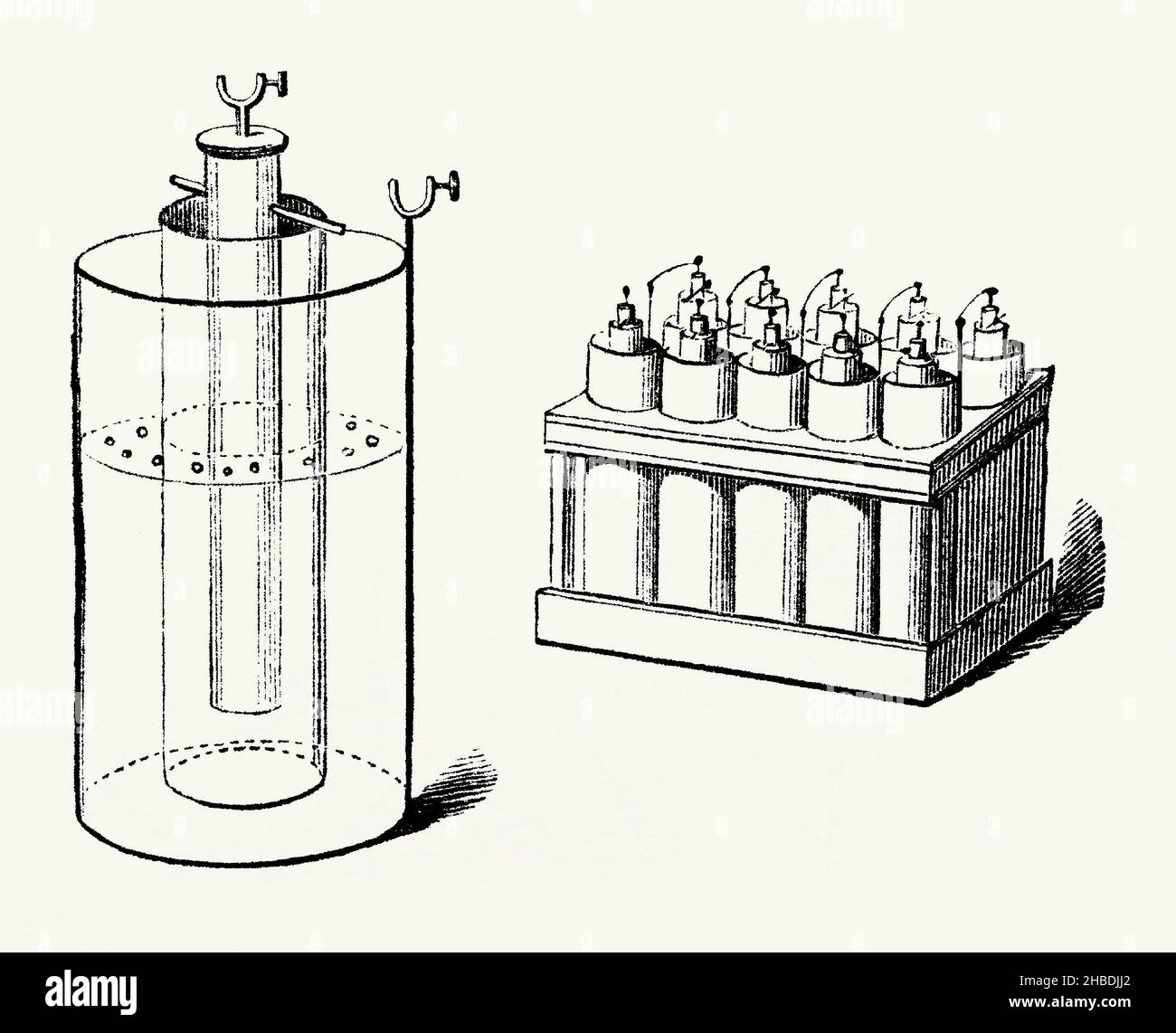 An old engraving of Daniell’s battery cell of 1836. It is from a book of the 1890s on discoveries and inventions during the 1800s. The cell is a type of electrochemical cell invented in 1836 by John Frederic Daniell (1790–1845), a British chemist and meteorologist, and consists of a copper pot filled with a copper sulphate solution, in which is immersed an unglazed earthenware container filled with sulphuric acid and a zinc electrode (a single cell is shown left, the battery right). It was a great improvement over the existing technology. Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/an-old-engraving-of-daniells-battery-cell-of-1836-it-is-from-a-book-of-the-1890s-on-discoveries-and-inventions-during-the-1800s-the-cell-is-a-type-of-electrochemical-cell-invented-in-1836-by-john-frederic-daniell-17901845-a-british-chemist-and-meteorologist-and-consists-of-a-copper-pot-filled-with-a-copper-sulphate-solution-in-which-is-immersed-an-unglazed-earthenware-container-filled-with-sulphuric-acid-and-a-zinc-electrode-a-single-cell-is-shown-left-the-battery-right-it-was-a-great-improvement-over-the-existing-technology-image454530778.html
An old engraving of Daniell’s battery cell of 1836. It is from a book of the 1890s on discoveries and inventions during the 1800s. The cell is a type of electrochemical cell invented in 1836 by John Frederic Daniell (1790–1845), a British chemist and meteorologist, and consists of a copper pot filled with a copper sulphate solution, in which is immersed an unglazed earthenware container filled with sulphuric acid and a zinc electrode (a single cell is shown left, the battery right). It was a great improvement over the existing technology. Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/an-old-engraving-of-daniells-battery-cell-of-1836-it-is-from-a-book-of-the-1890s-on-discoveries-and-inventions-during-the-1800s-the-cell-is-a-type-of-electrochemical-cell-invented-in-1836-by-john-frederic-daniell-17901845-a-british-chemist-and-meteorologist-and-consists-of-a-copper-pot-filled-with-a-copper-sulphate-solution-in-which-is-immersed-an-unglazed-earthenware-container-filled-with-sulphuric-acid-and-a-zinc-electrode-a-single-cell-is-shown-left-the-battery-right-it-was-a-great-improvement-over-the-existing-technology-image454530778.htmlRM2HBDJJ2–An old engraving of Daniell’s battery cell of 1836. It is from a book of the 1890s on discoveries and inventions during the 1800s. The cell is a type of electrochemical cell invented in 1836 by John Frederic Daniell (1790–1845), a British chemist and meteorologist, and consists of a copper pot filled with a copper sulphate solution, in which is immersed an unglazed earthenware container filled with sulphuric acid and a zinc electrode (a single cell is shown left, the battery right). It was a great improvement over the existing technology.
 Glass beaker containing copper strips connected to electrodes in copper sulphate solution (copper purification) Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/glass-beaker-containing-copper-strips-connected-to-electrodes-in-copper-sulphate-solution-copper-purification-image216246327.html
Glass beaker containing copper strips connected to electrodes in copper sulphate solution (copper purification) Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/glass-beaker-containing-copper-strips-connected-to-electrodes-in-copper-sulphate-solution-copper-purification-image216246327.htmlRMPFPTB3–Glass beaker containing copper strips connected to electrodes in copper sulphate solution (copper purification)
 Two tubes illustrating displacement of metal elements from solution Copper displaces the iron Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/two-tubes-illustrating-displacement-of-metal-elements-from-solution-image786198.html
Two tubes illustrating displacement of metal elements from solution Copper displaces the iron Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/two-tubes-illustrating-displacement-of-metal-elements-from-solution-image786198.htmlRMABFF16–Two tubes illustrating displacement of metal elements from solution Copper displaces the iron
 Close up of microscale electrolysis equipment in the process of copper sulphate electrolysis Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/close-up-of-microscale-electrolysis-equipment-in-the-process-of-copper-sulphate-electrolysis-image232865398.html
Close up of microscale electrolysis equipment in the process of copper sulphate electrolysis Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/close-up-of-microscale-electrolysis-equipment-in-the-process-of-copper-sulphate-electrolysis-image232865398.htmlRFRERX5A–Close up of microscale electrolysis equipment in the process of copper sulphate electrolysis
 Flask with copper sulfate solution Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/stock-photo-flask-with-copper-sulfate-solution-135729458.html
Flask with copper sulfate solution Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/stock-photo-flask-with-copper-sulfate-solution-135729458.htmlRFHTR08J–Flask with copper sulfate solution
 Making hydrogen gas by reacting, zinc metal with copper sulphate solution and sulphuric acid Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/making-hydrogen-gas-by-reacting-zinc-metal-with-copper-sulphate-solution-and-sulphuric-acid-image237289110.html
Making hydrogen gas by reacting, zinc metal with copper sulphate solution and sulphuric acid Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/making-hydrogen-gas-by-reacting-zinc-metal-with-copper-sulphate-solution-and-sulphuric-acid-image237289110.htmlRFRP1CK2–Making hydrogen gas by reacting, zinc metal with copper sulphate solution and sulphuric acid
 A farmer applying insecticides to his potato crop. Legs of a man in personal protective equipment for the application of pesticides. A man sprays potato bushes with a solution of copper sulphate. Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/a-farmer-applying-insecticides-to-his-potato-crop-legs-of-a-man-in-personal-protective-equipment-for-the-application-of-pesticides-a-man-sprays-potato-bushes-with-a-solution-of-copper-sulphate-image547798477.html
A farmer applying insecticides to his potato crop. Legs of a man in personal protective equipment for the application of pesticides. A man sprays potato bushes with a solution of copper sulphate. Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/a-farmer-applying-insecticides-to-his-potato-crop-legs-of-a-man-in-personal-protective-equipment-for-the-application-of-pesticides-a-man-sprays-potato-bushes-with-a-solution-of-copper-sulphate-image547798477.htmlRF2PR6AF9–A farmer applying insecticides to his potato crop. Legs of a man in personal protective equipment for the application of pesticides. A man sprays potato bushes with a solution of copper sulphate.
 Cathodes put into electrolytic solution to get copper powder Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/cathodes-put-into-electrolytic-solution-to-get-copper-powder-image485925437.html
Cathodes put into electrolytic solution to get copper powder Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/cathodes-put-into-electrolytic-solution-to-get-copper-powder-image485925437.htmlRF2K6FPRW–Cathodes put into electrolytic solution to get copper powder
 Making copper sulphate crystals in a UK school science experiment Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/making-copper-sulphate-crystals-in-a-uk-school-science-experiment-image344146499.html
Making copper sulphate crystals in a UK school science experiment Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/making-copper-sulphate-crystals-in-a-uk-school-science-experiment-image344146499.htmlRF2AYW6AB–Making copper sulphate crystals in a UK school science experiment
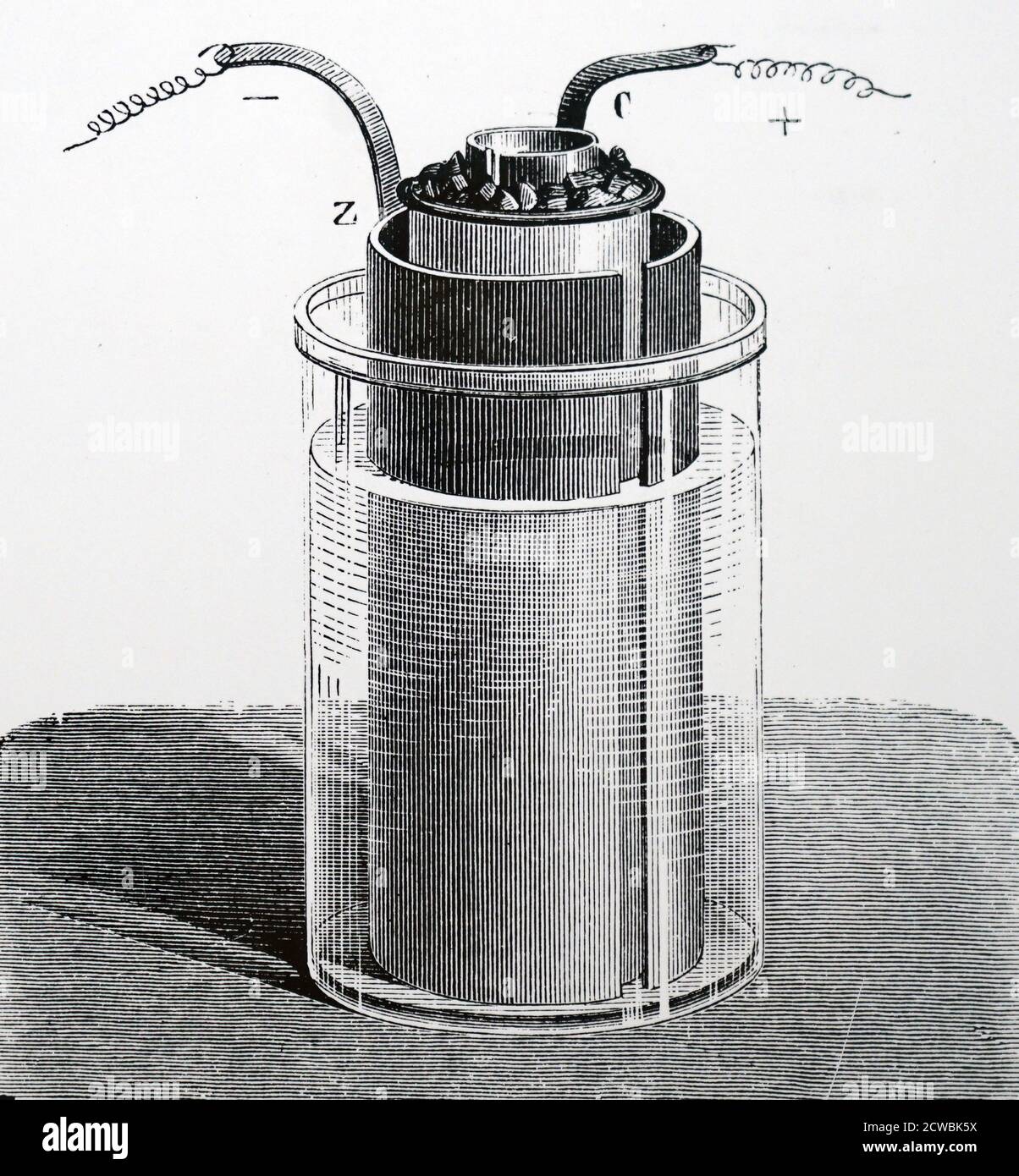 Engraving depicting John Frederic Daniell's element in a modified form. A glass vessel containing diluted sulphuric acid: inside is a zinc cylinder (Z) surrounding a porous vessel containing a copper tube and a solution of sulphate of copper. Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/engraving-depicting-john-frederic-daniells-element-in-a-modified-form-a-glass-vessel-containing-diluted-sulphuric-acid-inside-is-a-zinc-cylinder-z-surrounding-a-porous-vessel-containing-a-copper-tube-and-a-solution-of-sulphate-of-copper-image377040662.html
Engraving depicting John Frederic Daniell's element in a modified form. A glass vessel containing diluted sulphuric acid: inside is a zinc cylinder (Z) surrounding a porous vessel containing a copper tube and a solution of sulphate of copper. Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/engraving-depicting-john-frederic-daniells-element-in-a-modified-form-a-glass-vessel-containing-diluted-sulphuric-acid-inside-is-a-zinc-cylinder-z-surrounding-a-porous-vessel-containing-a-copper-tube-and-a-solution-of-sulphate-of-copper-image377040662.htmlRM2CWBK5X–Engraving depicting John Frederic Daniell's element in a modified form. A glass vessel containing diluted sulphuric acid: inside is a zinc cylinder (Z) surrounding a porous vessel containing a copper tube and a solution of sulphate of copper.
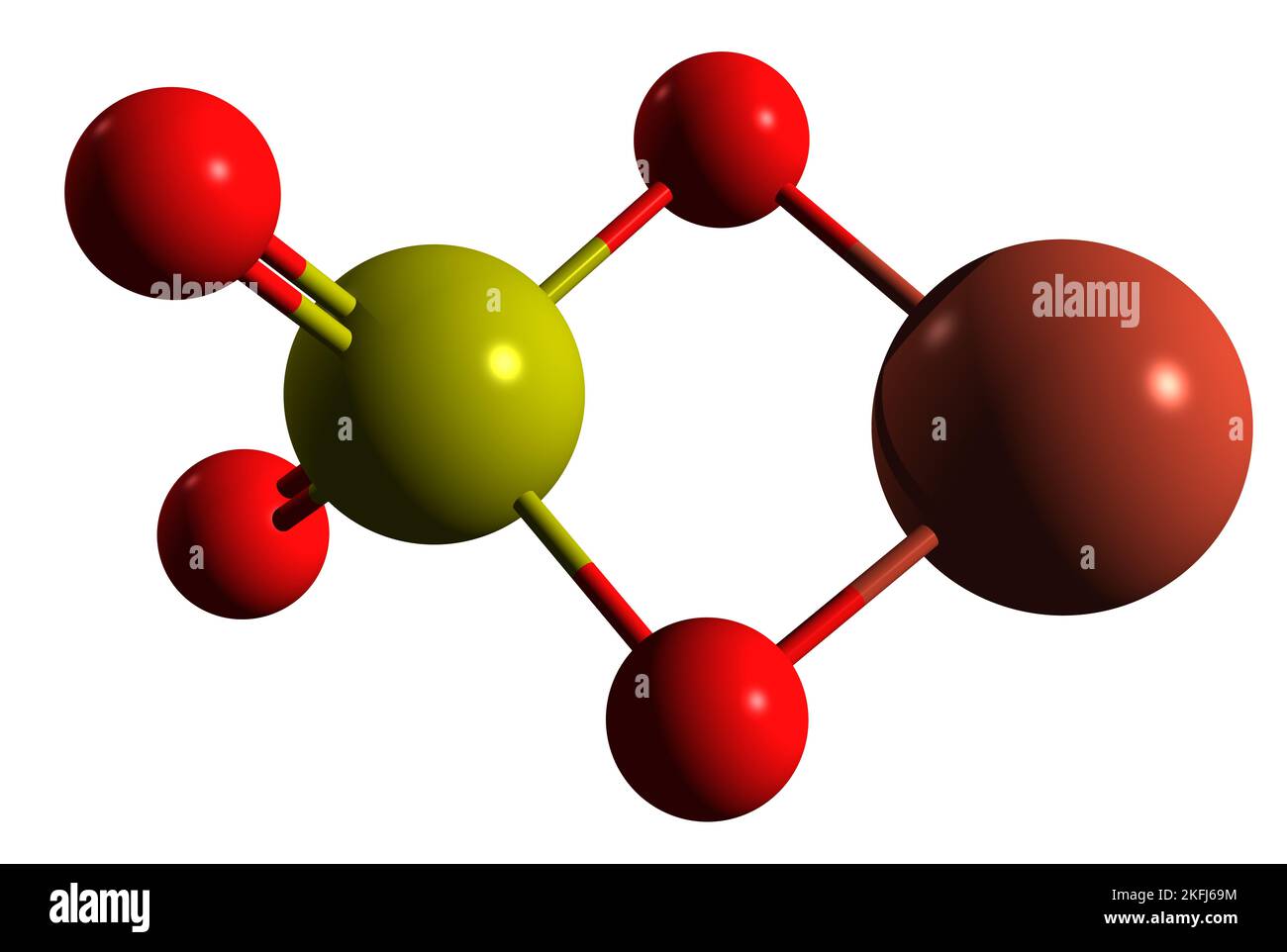 3D image of Copper II sulfate skeletal formula - molecular chemical structure of inorganic compound Blue vitriol isolated on white background Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/3d-image-of-copper-ii-sulfate-skeletal-formula-molecular-chemical-structure-of-inorganic-compound-blue-vitriol-isolated-on-white-background-image491510256.html
3D image of Copper II sulfate skeletal formula - molecular chemical structure of inorganic compound Blue vitriol isolated on white background Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/3d-image-of-copper-ii-sulfate-skeletal-formula-molecular-chemical-structure-of-inorganic-compound-blue-vitriol-isolated-on-white-background-image491510256.htmlRF2KFJ69M–3D image of Copper II sulfate skeletal formula - molecular chemical structure of inorganic compound Blue vitriol isolated on white background
 A copper sulphate crystal growing in a saturated solution in a glass as a school science project on crystallization Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/stock-photo-a-copper-sulphate-crystal-growing-in-a-saturated-solution-in-a-glass-73215210.html
A copper sulphate crystal growing in a saturated solution in a glass as a school science project on crystallization Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/stock-photo-a-copper-sulphate-crystal-growing-in-a-saturated-solution-in-a-glass-73215210.htmlRME736MX–A copper sulphate crystal growing in a saturated solution in a glass as a school science project on crystallization
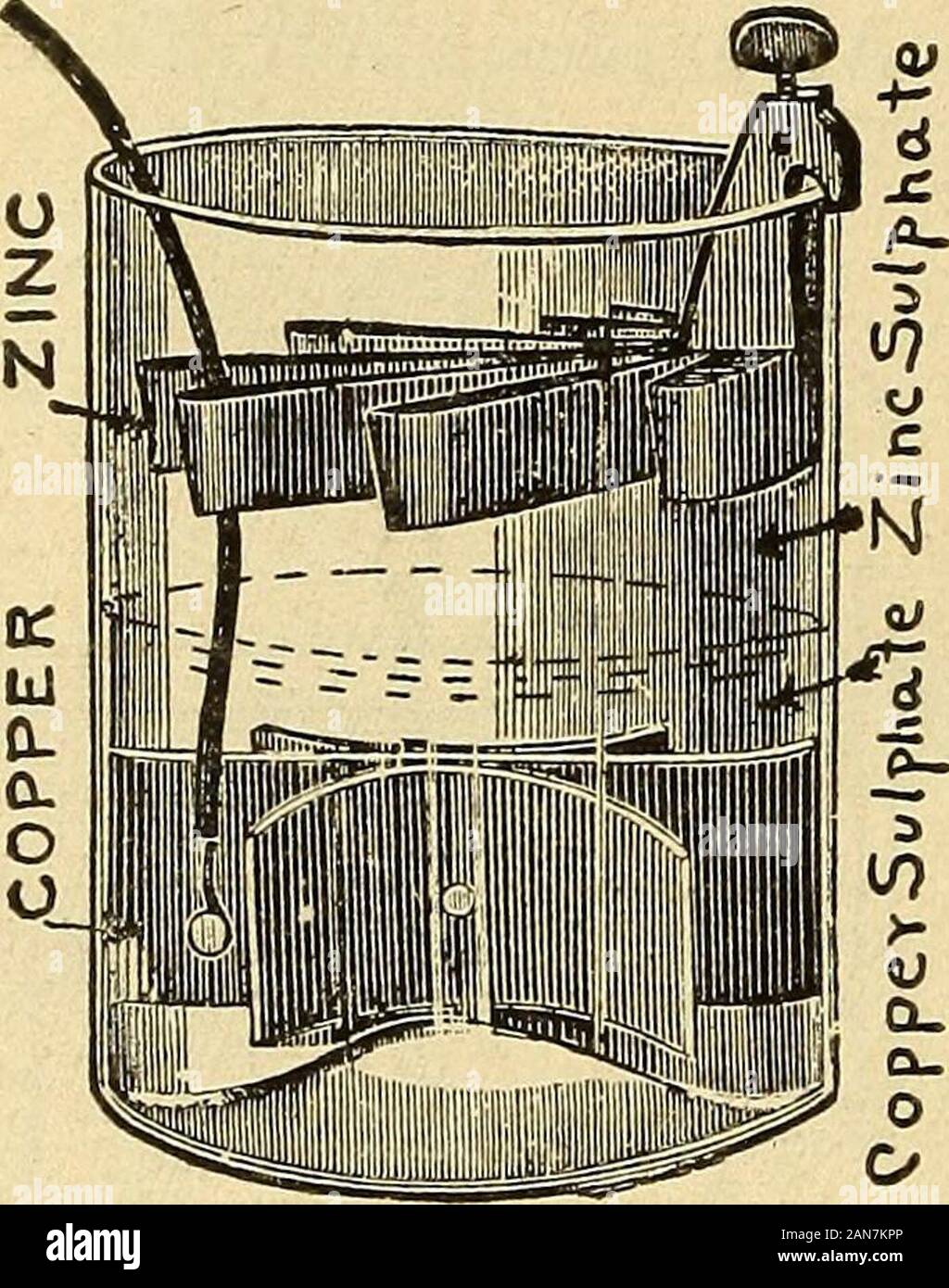 Lessons in practical electricity; principles, experiments, and arithmetical problems, an elementary text-book . immediate use; on the copper side is placedsulphate of copper (CuS04), dissolved in water, together withsome sulphate of copper crystals (bluestone) to maintain thesupply of copper sulphate solution. When the circuit isclosed, as shown by Fig. 84, the zinc combines with the(S0+) of the sulphuric acid forming sulphate of zinc(ZnSO ), and thus sets free the two molecules of hydrogen(H2). 0 PRACTICAL ELECTRICITY. This hydrogen gas passes through the porous partition,but instead of colle Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/lessons-in-practical-electricity-principles-experiments-and-arithmetical-problems-an-elementary-text-book-immediate-use-on-the-copper-side-is-placedsulphate-of-copper-cus04-dissolved-in-water-together-withsome-sulphate-of-copper-crystals-bluestone-to-maintain-thesupply-of-copper-sulphate-solution-when-the-circuit-isclosed-as-shown-by-fig-84-the-zinc-combines-with-thes0-of-the-sulphuric-acid-forming-sulphate-of-zincznso-and-thus-sets-free-the-two-molecules-of-hydrogenh2-0-practical-electricity-this-hydrogen-gas-passes-through-the-porous-partitionbut-instead-of-colle-image340073966.html
Lessons in practical electricity; principles, experiments, and arithmetical problems, an elementary text-book . immediate use; on the copper side is placedsulphate of copper (CuS04), dissolved in water, together withsome sulphate of copper crystals (bluestone) to maintain thesupply of copper sulphate solution. When the circuit isclosed, as shown by Fig. 84, the zinc combines with the(S0+) of the sulphuric acid forming sulphate of zinc(ZnSO ), and thus sets free the two molecules of hydrogen(H2). 0 PRACTICAL ELECTRICITY. This hydrogen gas passes through the porous partition,but instead of colle Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/lessons-in-practical-electricity-principles-experiments-and-arithmetical-problems-an-elementary-text-book-immediate-use-on-the-copper-side-is-placedsulphate-of-copper-cus04-dissolved-in-water-together-withsome-sulphate-of-copper-crystals-bluestone-to-maintain-thesupply-of-copper-sulphate-solution-when-the-circuit-isclosed-as-shown-by-fig-84-the-zinc-combines-with-thes0-of-the-sulphuric-acid-forming-sulphate-of-zincznso-and-thus-sets-free-the-two-molecules-of-hydrogenh2-0-practical-electricity-this-hydrogen-gas-passes-through-the-porous-partitionbut-instead-of-colle-image340073966.htmlRM2AN7KPP–Lessons in practical electricity; principles, experiments, and arithmetical problems, an elementary text-book . immediate use; on the copper side is placedsulphate of copper (CuS04), dissolved in water, together withsome sulphate of copper crystals (bluestone) to maintain thesupply of copper sulphate solution. When the circuit isclosed, as shown by Fig. 84, the zinc combines with the(S0+) of the sulphuric acid forming sulphate of zinc(ZnSO ), and thus sets free the two molecules of hydrogen(H2). 0 PRACTICAL ELECTRICITY. This hydrogen gas passes through the porous partition,but instead of colle
 Chemical reaction copper wire and argentum liquid Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/chemical-reaction-copper-wire-and-argentum-liquid-image379682019.html
Chemical reaction copper wire and argentum liquid Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/chemical-reaction-copper-wire-and-argentum-liquid-image379682019.htmlRF2D1M083–Chemical reaction copper wire and argentum liquid
 Abstract chemical copper background with chaotic pattern Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/abstract-chemical-copper-background-with-chaotic-pattern-image386535975.html
Abstract chemical copper background with chaotic pattern Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/abstract-chemical-copper-background-with-chaotic-pattern-image386535975.htmlRF2DCT6G7–Abstract chemical copper background with chaotic pattern
 an iron nail being removed from a beaker of copper sulphate solution after it has been copper plated it has displaced copper Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/an-iron-nail-being-removed-from-a-beaker-of-copper-sulphate-solution-image1418024.html
an iron nail being removed from a beaker of copper sulphate solution after it has been copper plated it has displaced copper Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/an-iron-nail-being-removed-from-a-beaker-of-copper-sulphate-solution-image1418024.htmlRMANA329–an iron nail being removed from a beaker of copper sulphate solution after it has been copper plated it has displaced copper
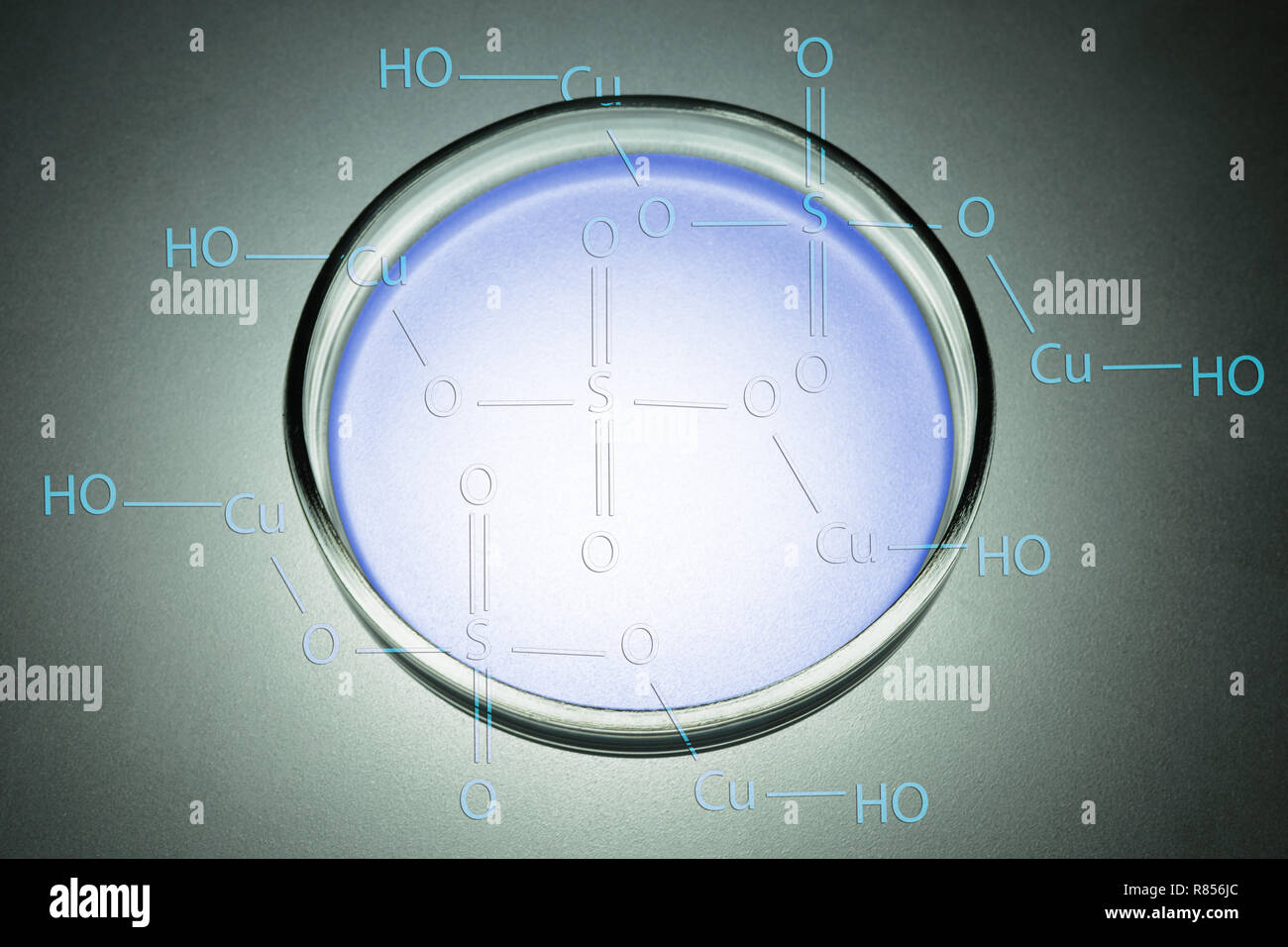 Abstract of petri dishes containing copper sulfate solution. Chemical formula. Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/abstract-of-petri-dishes-containing-copper-sulfate-solution-chemical-formula-image228767012.html
Abstract of petri dishes containing copper sulfate solution. Chemical formula. Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/abstract-of-petri-dishes-containing-copper-sulfate-solution-chemical-formula-image228767012.htmlRFR856JC–Abstract of petri dishes containing copper sulfate solution. Chemical formula.
 Petri dishes of copper sulphate solution isolated on white background Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/stock-image-petri-dishes-of-copper-sulphate-solution-isolated-on-white-background-162029293.html
Petri dishes of copper sulphate solution isolated on white background Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/stock-image-petri-dishes-of-copper-sulphate-solution-isolated-on-white-background-162029293.htmlRFKBH20D–Petri dishes of copper sulphate solution isolated on white background
 Single replacement reaction, illustration Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/single-replacement-reaction-illustration-image328920545.html
Single replacement reaction, illustration Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/single-replacement-reaction-illustration-image328920545.htmlRF2A33HE9–Single replacement reaction, illustration
 Glass ampoules containing iron supplement solution to treat anaemia. Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/glass-ampoules-containing-iron-supplement-solution-to-treat-anaemia-image470064300.html
Glass ampoules containing iron supplement solution to treat anaemia. Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/glass-ampoules-containing-iron-supplement-solution-to-treat-anaemia-image470064300.htmlRF2J8N7PM–Glass ampoules containing iron supplement solution to treat anaemia.
 Solid metallic copper at bottom of glass beaker containing Zinc sulphate solution, beaker of blue copper sulphate solution, and zinc metal shavings on watchglass Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/solid-metallic-copper-at-bottom-of-glass-beaker-containing-zinc-sulphate-solution-beaker-of-blue-copper-sulphate-solution-and-zinc-metal-shavings-on-watchglass-image216120239.html
Solid metallic copper at bottom of glass beaker containing Zinc sulphate solution, beaker of blue copper sulphate solution, and zinc metal shavings on watchglass Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/solid-metallic-copper-at-bottom-of-glass-beaker-containing-zinc-sulphate-solution-beaker-of-blue-copper-sulphate-solution-and-zinc-metal-shavings-on-watchglass-image216120239.htmlRMPFH3FY–Solid metallic copper at bottom of glass beaker containing Zinc sulphate solution, beaker of blue copper sulphate solution, and zinc metal shavings on watchglass
 School science laboratory with bottles of chemicals ready for an experiment, a bottle of potassium chromate solution in front. Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/stock-photo-school-science-laboratory-with-bottles-of-chemicals-ready-for-an-experiment-30365527.html
School science laboratory with bottles of chemicals ready for an experiment, a bottle of potassium chromate solution in front. Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/stock-photo-school-science-laboratory-with-bottles-of-chemicals-ready-for-an-experiment-30365527.htmlRMBNB7F3–School science laboratory with bottles of chemicals ready for an experiment, a bottle of potassium chromate solution in front.
 Battery connected to a copper pipe and a key in copper sulphate solution, copper plating. Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/stock-photo-battery-connected-to-a-copper-pipe-and-a-key-in-copper-sulphate-solution-95273838.html
Battery connected to a copper pipe and a key in copper sulphate solution, copper plating. Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/stock-photo-battery-connected-to-a-copper-pipe-and-a-key-in-copper-sulphate-solution-95273838.htmlRMFF02N2–Battery connected to a copper pipe and a key in copper sulphate solution, copper plating.
 Crystal and water solution of copper sulphate Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/stock-photo-crystal-and-water-solution-of-copper-sulphate-129127624.html
Crystal and water solution of copper sulphate Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/stock-photo-crystal-and-water-solution-of-copper-sulphate-129127624.htmlRFHE27GT–Crystal and water solution of copper sulphate
 Experiment showing crystal formation, copper (II) sulphate solution after one hour Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/experiment-showing-crystal-formation-copper-ii-sulphate-solution-after-one-hour-image216189547.html
Experiment showing crystal formation, copper (II) sulphate solution after one hour Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/experiment-showing-crystal-formation-copper-ii-sulphate-solution-after-one-hour-image216189547.htmlRMPFM7Y7–Experiment showing crystal formation, copper (II) sulphate solution after one hour
 A farmer applying insecticides to his potato crop. Legs of a man in personal protective equipment for the application of pesticides. A man sprays potato bushes with a solution of copper sulphate. Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/a-farmer-applying-insecticides-to-his-potato-crop-legs-of-a-man-in-personal-protective-equipment-for-the-application-of-pesticides-a-man-sprays-potato-bushes-with-a-solution-of-copper-sulphate-image547798461.html
A farmer applying insecticides to his potato crop. Legs of a man in personal protective equipment for the application of pesticides. A man sprays potato bushes with a solution of copper sulphate. Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/a-farmer-applying-insecticides-to-his-potato-crop-legs-of-a-man-in-personal-protective-equipment-for-the-application-of-pesticides-a-man-sprays-potato-bushes-with-a-solution-of-copper-sulphate-image547798461.htmlRF2PR6AEN–A farmer applying insecticides to his potato crop. Legs of a man in personal protective equipment for the application of pesticides. A man sprays potato bushes with a solution of copper sulphate.
 Cathodes put into electrolytic solution to get copper powder Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/cathodes-put-into-electrolytic-solution-to-get-copper-powder-image490742308.html
Cathodes put into electrolytic solution to get copper powder Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/cathodes-put-into-electrolytic-solution-to-get-copper-powder-image490742308.htmlRF2KEB6R0–Cathodes put into electrolytic solution to get copper powder
 medicinally medical algae vessel copper laboratory solution watchword sulphate Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/stock-photo-medicinally-medical-algae-vessel-copper-laboratory-solution-watchword-141111041.html
medicinally medical algae vessel copper laboratory solution watchword sulphate Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/stock-photo-medicinally-medical-algae-vessel-copper-laboratory-solution-watchword-141111041.htmlRFJ5G4G1–medicinally medical algae vessel copper laboratory solution watchword sulphate
 Making copper sulphate crystals in a UK school science experiment Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/making-copper-sulphate-crystals-in-a-uk-school-science-experiment-image344146422.html
Making copper sulphate crystals in a UK school science experiment Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/making-copper-sulphate-crystals-in-a-uk-school-science-experiment-image344146422.htmlRF2AYW67J–Making copper sulphate crystals in a UK school science experiment
 A scientist adding drops of a solution from a pipette into a conical flask with a blue solution Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/a-scientist-adding-drops-of-a-solution-from-a-pipette-into-a-conical-image66145937.html
A scientist adding drops of a solution from a pipette into a conical flask with a blue solution Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/a-scientist-adding-drops-of-a-solution-from-a-pipette-into-a-conical-image66145937.htmlRFDRH5PW–A scientist adding drops of a solution from a pipette into a conical flask with a blue solution
 full frame background and texture of blue copper sulfate granules - close-up. Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/full-frame-background-and-texture-of-blue-copper-sulfate-granules-close-up-image398343542.html
full frame background and texture of blue copper sulfate granules - close-up. Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/full-frame-background-and-texture-of-blue-copper-sulfate-granules-close-up-image398343542.htmlRF2E42372–full frame background and texture of blue copper sulfate granules - close-up.
 Laboratory exercises to accompany Carhart and Chute's First principles of physics . ype into the wax,until a clean-cut impressionextends nearly but not quitethrough the wax, when the typeis removed. Dust the impres-sion again with graphite, tak-ing care not to mar the outline. With a brush and meltedbeeswax, coat the back andedges of the lead strip up to thepoint where it is to be clamped. Clamp the lead and copperstrips in place, so that theimpression is toward the copper strip. Immerse thestrips in a tumbler of copper sulphate solution. Theelectrodes should be about one centimeter apart. Ar- Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/laboratory-exercises-to-accompany-carhart-and-chutes-first-principles-of-physics-ype-into-the-waxuntil-a-clean-cut-impressionextends-nearly-but-not-quitethrough-the-wax-when-the-typeis-removed-dust-the-impres-sion-again-with-graphite-tak-ing-care-not-to-mar-the-outline-with-a-brush-and-meltedbeeswax-coat-the-back-andedges-of-the-lead-strip-up-to-thepoint-where-it-is-to-be-clamped-clamp-the-lead-and-copperstrips-in-place-so-that-theimpression-is-toward-the-copper-strip-immerse-thestrips-in-a-tumbler-of-copper-sulphate-solution-theelectrodes-should-be-about-one-centimeter-apart-ar-image340306430.html
Laboratory exercises to accompany Carhart and Chute's First principles of physics . ype into the wax,until a clean-cut impressionextends nearly but not quitethrough the wax, when the typeis removed. Dust the impres-sion again with graphite, tak-ing care not to mar the outline. With a brush and meltedbeeswax, coat the back andedges of the lead strip up to thepoint where it is to be clamped. Clamp the lead and copperstrips in place, so that theimpression is toward the copper strip. Immerse thestrips in a tumbler of copper sulphate solution. Theelectrodes should be about one centimeter apart. Ar- Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/laboratory-exercises-to-accompany-carhart-and-chutes-first-principles-of-physics-ype-into-the-waxuntil-a-clean-cut-impressionextends-nearly-but-not-quitethrough-the-wax-when-the-typeis-removed-dust-the-impres-sion-again-with-graphite-tak-ing-care-not-to-mar-the-outline-with-a-brush-and-meltedbeeswax-coat-the-back-andedges-of-the-lead-strip-up-to-thepoint-where-it-is-to-be-clamped-clamp-the-lead-and-copperstrips-in-place-so-that-theimpression-is-toward-the-copper-strip-immerse-thestrips-in-a-tumbler-of-copper-sulphate-solution-theelectrodes-should-be-about-one-centimeter-apart-ar-image340306430.htmlRM2ANJ892–Laboratory exercises to accompany Carhart and Chute's First principles of physics . ype into the wax,until a clean-cut impressionextends nearly but not quitethrough the wax, when the typeis removed. Dust the impres-sion again with graphite, tak-ing care not to mar the outline. With a brush and meltedbeeswax, coat the back andedges of the lead strip up to thepoint where it is to be clamped. Clamp the lead and copperstrips in place, so that theimpression is toward the copper strip. Immerse thestrips in a tumbler of copper sulphate solution. Theelectrodes should be about one centimeter apart. Ar-
 . The encyclopedia of practical horticulture; a reference system of commercial horticulture, covering the practical and scientific phases of horticulture, with special reference to fruits and vegetables;. Gardening; Fruit-culture; Vegetable gardening. CUCUMBER DISEASES—CUCUMBER PESTS 857 Massachusetts Experiment Station Bulle- tin 55. In thus handling the soil due time must be given for draining and dry- ^^^' A. D. Selby Powdery Mildew Erysiphe cichoracearum DC. Frequent in hothouses, but not trouble- some elsewhere. Selby recommends a dilute copper sulphate solution. Keference Ohio Experiment Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/the-encyclopedia-of-practical-horticulture-a-reference-system-of-commercial-horticulture-covering-the-practical-and-scientific-phases-of-horticulture-with-special-reference-to-fruits-and-vegetables-gardening-fruit-culture-vegetable-gardening-cucumber-diseasescucumber-pests-857-massachusetts-experiment-station-bulle-tin-55-in-thus-handling-the-soil-due-time-must-be-given-for-draining-and-dry-a-d-selby-powdery-mildew-erysiphe-cichoracearum-dc-frequent-in-hothouses-but-not-trouble-some-elsewhere-selby-recommends-a-dilute-copper-sulphate-solution-keference-ohio-experiment-image216338076.html
. The encyclopedia of practical horticulture; a reference system of commercial horticulture, covering the practical and scientific phases of horticulture, with special reference to fruits and vegetables;. Gardening; Fruit-culture; Vegetable gardening. CUCUMBER DISEASES—CUCUMBER PESTS 857 Massachusetts Experiment Station Bulle- tin 55. In thus handling the soil due time must be given for draining and dry- ^^^' A. D. Selby Powdery Mildew Erysiphe cichoracearum DC. Frequent in hothouses, but not trouble- some elsewhere. Selby recommends a dilute copper sulphate solution. Keference Ohio Experiment Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/the-encyclopedia-of-practical-horticulture-a-reference-system-of-commercial-horticulture-covering-the-practical-and-scientific-phases-of-horticulture-with-special-reference-to-fruits-and-vegetables-gardening-fruit-culture-vegetable-gardening-cucumber-diseasescucumber-pests-857-massachusetts-experiment-station-bulle-tin-55-in-thus-handling-the-soil-due-time-must-be-given-for-draining-and-dry-a-d-selby-powdery-mildew-erysiphe-cichoracearum-dc-frequent-in-hothouses-but-not-trouble-some-elsewhere-selby-recommends-a-dilute-copper-sulphate-solution-keference-ohio-experiment-image216338076.htmlRMPFY1BT–. The encyclopedia of practical horticulture; a reference system of commercial horticulture, covering the practical and scientific phases of horticulture, with special reference to fruits and vegetables;. Gardening; Fruit-culture; Vegetable gardening. CUCUMBER DISEASES—CUCUMBER PESTS 857 Massachusetts Experiment Station Bulle- tin 55. In thus handling the soil due time must be given for draining and dry- ^^^' A. D. Selby Powdery Mildew Erysiphe cichoracearum DC. Frequent in hothouses, but not trouble- some elsewhere. Selby recommends a dilute copper sulphate solution. Keference Ohio Experiment
 Spraying an apple tree with fruits from codling moth and aphids. Treatment of apple trees with copper sulphate and ammonia. Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/spraying-an-apple-tree-with-fruits-from-codling-moth-and-aphids-treatment-of-apple-trees-with-copper-sulphate-and-ammonia-image541445291.html
Spraying an apple tree with fruits from codling moth and aphids. Treatment of apple trees with copper sulphate and ammonia. Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/spraying-an-apple-tree-with-fruits-from-codling-moth-and-aphids-treatment-of-apple-trees-with-copper-sulphate-and-ammonia-image541445291.htmlRF2PCTXYR–Spraying an apple tree with fruits from codling moth and aphids. Treatment of apple trees with copper sulphate and ammonia.
 An iron nail in copper sulphate solution becomes coated with copper Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/stock-photo-an-iron-nail-in-copper-sulphate-solution-becomes-coated-with-copper-19453831.html
An iron nail in copper sulphate solution becomes coated with copper Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/stock-photo-an-iron-nail-in-copper-sulphate-solution-becomes-coated-with-copper-19453831.htmlRMB3J5FK–An iron nail in copper sulphate solution becomes coated with copper
 chemical blue earth Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/chemical-blue-earth-image479312878.html
chemical blue earth Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/chemical-blue-earth-image479312878.htmlRF2JRPGD2–chemical blue earth
 Petri dishes of copper sulphate solution isolated on white background Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/stock-image-petri-dishes-of-copper-sulphate-solution-isolated-on-white-background-162029344.html
Petri dishes of copper sulphate solution isolated on white background Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/stock-image-petri-dishes-of-copper-sulphate-solution-isolated-on-white-background-162029344.htmlRFKBH228–Petri dishes of copper sulphate solution isolated on white background
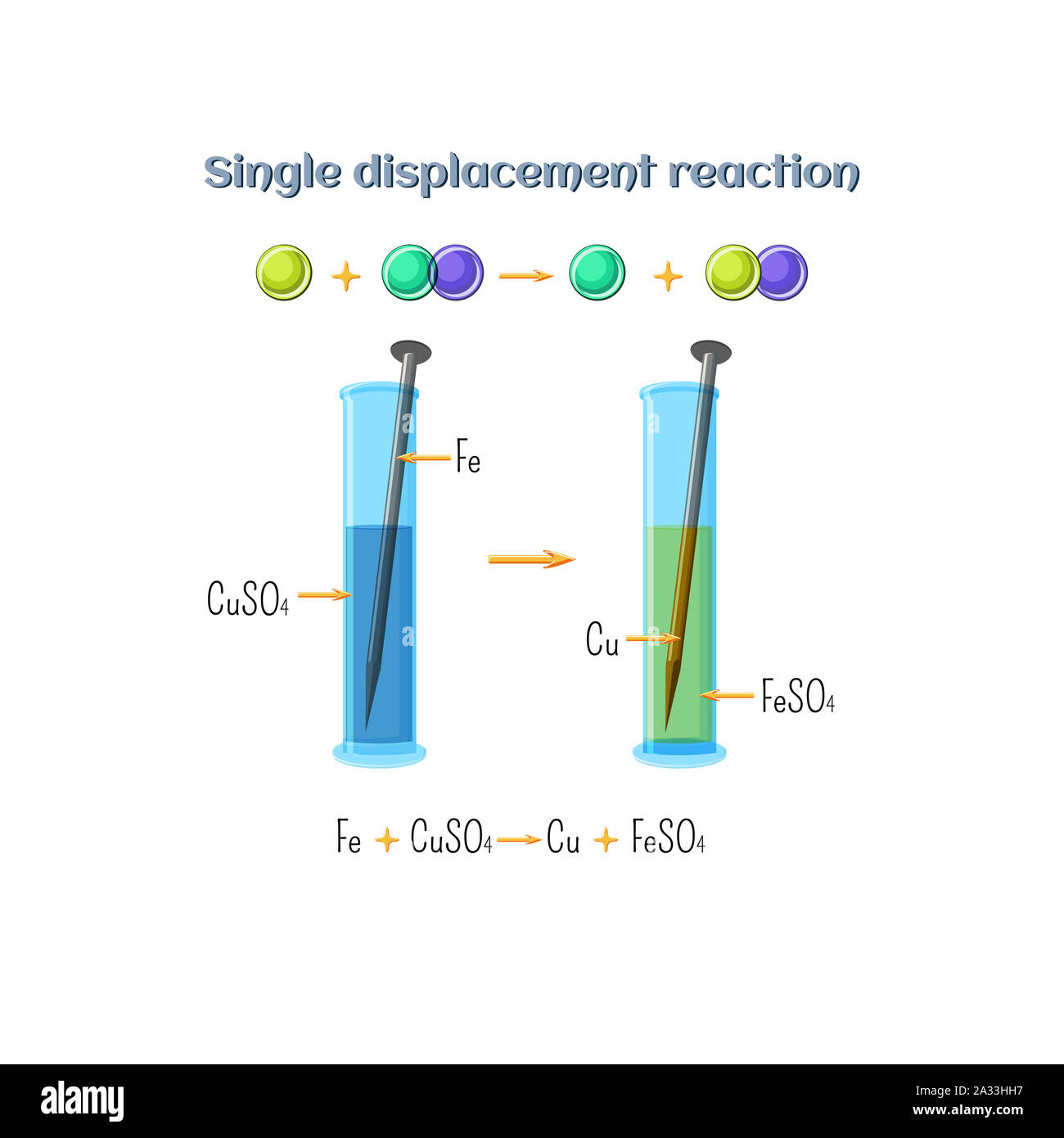 Single displacement reaction, illustration Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/single-displacement-reaction-illustration-image328920627.html
Single displacement reaction, illustration Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/single-displacement-reaction-illustration-image328920627.htmlRF2A33HH7–Single displacement reaction, illustration
 Glass ampoules containing iron supplement solution to treat anaemia. Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/glass-ampoules-containing-iron-supplement-solution-to-treat-anaemia-image470064756.html
Glass ampoules containing iron supplement solution to treat anaemia. Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/glass-ampoules-containing-iron-supplement-solution-to-treat-anaemia-image470064756.htmlRF2J8N8B0–Glass ampoules containing iron supplement solution to treat anaemia.
 processes to the society does not afford them the pro tection of a patent which should be applied for before the competition. for an excellent disinfectant: Four pounds of crude sulphate of iron or two pounds of sulphate of copper are dissolved in hot water to which two ounces of sul phuric acid are added. Mix witb the Solution while bottles. When this powerful remedy cannot be ap plied in its fluid state dry sawdukt thorougbly moist ened with it may be scattered over the Boor of a dark room or other places to be disinfected, scientific american, 1887-01-15 Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/processes-to-the-society-does-not-afford-them-the-pro-tection-of-a-patent-which-should-be-applied-for-before-the-competition-for-an-excellent-disinfectant-four-pounds-of-crude-sulphate-of-iron-or-two-pounds-of-sulphate-of-copper-are-dissolved-in-hot-water-to-which-two-ounces-of-sul-phuric-acid-are-added-mix-witb-the-solution-while-bottles-when-this-powerful-remedy-cannot-be-ap-plied-in-its-fluid-state-dry-sawdukt-thorougbly-moist-ened-with-it-may-be-scattered-over-the-boor-of-a-dark-room-or-other-places-to-be-disinfected-scientific-american-1887-01-15-image334330232.html
processes to the society does not afford them the pro tection of a patent which should be applied for before the competition. for an excellent disinfectant: Four pounds of crude sulphate of iron or two pounds of sulphate of copper are dissolved in hot water to which two ounces of sul phuric acid are added. Mix witb the Solution while bottles. When this powerful remedy cannot be ap plied in its fluid state dry sawdukt thorougbly moist ened with it may be scattered over the Boor of a dark room or other places to be disinfected, scientific american, 1887-01-15 Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/processes-to-the-society-does-not-afford-them-the-pro-tection-of-a-patent-which-should-be-applied-for-before-the-competition-for-an-excellent-disinfectant-four-pounds-of-crude-sulphate-of-iron-or-two-pounds-of-sulphate-of-copper-are-dissolved-in-hot-water-to-which-two-ounces-of-sul-phuric-acid-are-added-mix-witb-the-solution-while-bottles-when-this-powerful-remedy-cannot-be-ap-plied-in-its-fluid-state-dry-sawdukt-thorougbly-moist-ened-with-it-may-be-scattered-over-the-boor-of-a-dark-room-or-other-places-to-be-disinfected-scientific-american-1887-01-15-image334330232.htmlRM2ABX1HC–processes to the society does not afford them the pro tection of a patent which should be applied for before the competition. for an excellent disinfectant: Four pounds of crude sulphate of iron or two pounds of sulphate of copper are dissolved in hot water to which two ounces of sul phuric acid are added. Mix witb the Solution while bottles. When this powerful remedy cannot be ap plied in its fluid state dry sawdukt thorougbly moist ened with it may be scattered over the Boor of a dark room or other places to be disinfected, scientific american, 1887-01-15
![Diseases of cultivated plants and Diseases of cultivated plants and trees diseasesofcultiv00massuoft Year: [1910?] SPHAERELLA 193 which should consequently be cleared out and spread over grass land. If in a house, after the soil has been removed, every part of the structure should be thoroughly drenched with a solution of sulphate of copper—one pound to fifteen Fig. 51.—Hypomyces perniciosKs. i, mushrooms deformed by the fungus, half nat. size ; 2, conidia of the fungus, highly mag. gallons of water; this should be repeated twice at an interval of about three weeks. Jour71. Bd. Agric. Lea Stock Photo Diseases of cultivated plants and Diseases of cultivated plants and trees diseasesofcultiv00massuoft Year: [1910?] SPHAERELLA 193 which should consequently be cleared out and spread over grass land. If in a house, after the soil has been removed, every part of the structure should be thoroughly drenched with a solution of sulphate of copper—one pound to fifteen Fig. 51.—Hypomyces perniciosKs. i, mushrooms deformed by the fungus, half nat. size ; 2, conidia of the fungus, highly mag. gallons of water; this should be repeated twice at an interval of about three weeks. Jour71. Bd. Agric. Lea Stock Photo](https://c8.alamy.com/comp/T1HHTK/diseases-of-cultivated-plants-and-diseases-of-cultivated-plants-and-trees-diseasesofcultiv00massuoft-year-1910-sphaerella-193-which-should-consequently-be-cleared-out-and-spread-over-grass-land-if-in-a-house-after-the-soil-has-been-removed-every-part-of-the-structure-should-be-thoroughly-drenched-with-a-solution-of-sulphate-of-copperone-pound-to-fifteen-fig-51hypomyces-perniciosks-i-mushrooms-deformed-by-the-fungus-half-nat-size-2-conidia-of-the-fungus-highly-mag-gallons-of-water-this-should-be-repeated-twice-at-an-interval-of-about-three-weeks-jour71-bd-agric-lea-T1HHTK.jpg) Diseases of cultivated plants and Diseases of cultivated plants and trees diseasesofcultiv00massuoft Year: [1910?] SPHAERELLA 193 which should consequently be cleared out and spread over grass land. If in a house, after the soil has been removed, every part of the structure should be thoroughly drenched with a solution of sulphate of copper—one pound to fifteen Fig. 51.—Hypomyces perniciosKs. i, mushrooms deformed by the fungus, half nat. size ; 2, conidia of the fungus, highly mag. gallons of water; this should be repeated twice at an interval of about three weeks. Jour71. Bd. Agric. Lea Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/diseases-of-cultivated-plants-and-diseases-of-cultivated-plants-and-trees-diseasesofcultiv00massuoft-year-1910-sphaerella-193-which-should-consequently-be-cleared-out-and-spread-over-grass-land-if-in-a-house-after-the-soil-has-been-removed-every-part-of-the-structure-should-be-thoroughly-drenched-with-a-solution-of-sulphate-of-copperone-pound-to-fifteen-fig-51hypomyces-perniciosks-i-mushrooms-deformed-by-the-fungus-half-nat-size-2-conidia-of-the-fungus-highly-mag-gallons-of-water-this-should-be-repeated-twice-at-an-interval-of-about-three-weeks-jour71-bd-agric-lea-image241947011.html
Diseases of cultivated plants and Diseases of cultivated plants and trees diseasesofcultiv00massuoft Year: [1910?] SPHAERELLA 193 which should consequently be cleared out and spread over grass land. If in a house, after the soil has been removed, every part of the structure should be thoroughly drenched with a solution of sulphate of copper—one pound to fifteen Fig. 51.—Hypomyces perniciosKs. i, mushrooms deformed by the fungus, half nat. size ; 2, conidia of the fungus, highly mag. gallons of water; this should be repeated twice at an interval of about three weeks. Jour71. Bd. Agric. Lea Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/diseases-of-cultivated-plants-and-diseases-of-cultivated-plants-and-trees-diseasesofcultiv00massuoft-year-1910-sphaerella-193-which-should-consequently-be-cleared-out-and-spread-over-grass-land-if-in-a-house-after-the-soil-has-been-removed-every-part-of-the-structure-should-be-thoroughly-drenched-with-a-solution-of-sulphate-of-copperone-pound-to-fifteen-fig-51hypomyces-perniciosks-i-mushrooms-deformed-by-the-fungus-half-nat-size-2-conidia-of-the-fungus-highly-mag-gallons-of-water-this-should-be-repeated-twice-at-an-interval-of-about-three-weeks-jour71-bd-agric-lea-image241947011.htmlRMT1HHTK–Diseases of cultivated plants and Diseases of cultivated plants and trees diseasesofcultiv00massuoft Year: [1910?] SPHAERELLA 193 which should consequently be cleared out and spread over grass land. If in a house, after the soil has been removed, every part of the structure should be thoroughly drenched with a solution of sulphate of copper—one pound to fifteen Fig. 51.—Hypomyces perniciosKs. i, mushrooms deformed by the fungus, half nat. size ; 2, conidia of the fungus, highly mag. gallons of water; this should be repeated twice at an interval of about three weeks. Jour71. Bd. Agric. Lea
 . Fig. 92.—Plu-agmidium stibcorticatum. i, rose branch and leaves with aecidium stage of fungus ; 2, rose leaf with teleutospores ; 3, teleutospores ; 4, uredospores. Figs, i and 2 nat. size, remainder highly mag. been attacked should be drenched with a solution of sulphate of copper during the winter. Raspberry rust {Phragmidium rubi-idaei, Winter) pro- duces its three stages on the raspberry plant. The aecidium condition appears first on the upper surface of the leaves in the month of June, under the form of greenish-yellow pustules, usually arranged in broken circles. The uredo stage appear Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/fig-92plu-agmidium-stibcorticatum-i-rose-branch-and-leaves-with-aecidium-stage-of-fungus-2-rose-leaf-with-teleutospores-3-teleutospores-4-uredospores-figs-i-and-2-nat-size-remainder-highly-mag-been-attacked-should-be-drenched-with-a-solution-of-sulphate-of-copper-during-the-winter-raspberry-rust-phragmidium-rubi-idaei-winter-pro-duces-its-three-stages-on-the-raspberry-plant-the-aecidium-condition-appears-first-on-the-upper-surface-of-the-leaves-in-the-month-of-june-under-the-form-of-greenish-yellow-pustules-usually-arranged-in-broken-circles-the-uredo-stage-appear-image179906351.html
. Fig. 92.—Plu-agmidium stibcorticatum. i, rose branch and leaves with aecidium stage of fungus ; 2, rose leaf with teleutospores ; 3, teleutospores ; 4, uredospores. Figs, i and 2 nat. size, remainder highly mag. been attacked should be drenched with a solution of sulphate of copper during the winter. Raspberry rust {Phragmidium rubi-idaei, Winter) pro- duces its three stages on the raspberry plant. The aecidium condition appears first on the upper surface of the leaves in the month of June, under the form of greenish-yellow pustules, usually arranged in broken circles. The uredo stage appear Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/fig-92plu-agmidium-stibcorticatum-i-rose-branch-and-leaves-with-aecidium-stage-of-fungus-2-rose-leaf-with-teleutospores-3-teleutospores-4-uredospores-figs-i-and-2-nat-size-remainder-highly-mag-been-attacked-should-be-drenched-with-a-solution-of-sulphate-of-copper-during-the-winter-raspberry-rust-phragmidium-rubi-idaei-winter-pro-duces-its-three-stages-on-the-raspberry-plant-the-aecidium-condition-appears-first-on-the-upper-surface-of-the-leaves-in-the-month-of-june-under-the-form-of-greenish-yellow-pustules-usually-arranged-in-broken-circles-the-uredo-stage-appear-image179906351.htmlRMMCKCAR–. Fig. 92.—Plu-agmidium stibcorticatum. i, rose branch and leaves with aecidium stage of fungus ; 2, rose leaf with teleutospores ; 3, teleutospores ; 4, uredospores. Figs, i and 2 nat. size, remainder highly mag. been attacked should be drenched with a solution of sulphate of copper during the winter. Raspberry rust {Phragmidium rubi-idaei, Winter) pro- duces its three stages on the raspberry plant. The aecidium condition appears first on the upper surface of the leaves in the month of June, under the form of greenish-yellow pustules, usually arranged in broken circles. The uredo stage appear
 Copper sulfate - crystal and water solution Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/stock-photo-copper-sulfate-crystal-and-water-solution-129127623.html
Copper sulfate - crystal and water solution Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/stock-photo-copper-sulfate-crystal-and-water-solution-129127623.htmlRFHE27GR–Copper sulfate - crystal and water solution
 Flasks with dissolved inorganic salts - potassium permanganate and copper sulphate Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/stock-photo-flasks-with-dissolved-inorganic-salts-potassium-permanganate-and-copper-129898654.html
Flasks with dissolved inorganic salts - potassium permanganate and copper sulphate Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/stock-photo-flasks-with-dissolved-inorganic-salts-potassium-permanganate-and-copper-129898654.htmlRFHF9B1J–Flasks with dissolved inorganic salts - potassium permanganate and copper sulphate
 Cathodes put into electrolytic solution to get copper powder Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/cathodes-put-into-electrolytic-solution-to-get-copper-powder-image474250690.html
Cathodes put into electrolytic solution to get copper powder Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/cathodes-put-into-electrolytic-solution-to-get-copper-powder-image474250690.htmlRF2JFFYGJ–Cathodes put into electrolytic solution to get copper powder
 blue medicinally medical industry algae vessel copper turquoise means agent Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/stock-photo-blue-medicinally-medical-industry-algae-vessel-copper-turquoise-means-141103166.html
blue medicinally medical industry algae vessel copper turquoise means agent Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/stock-photo-blue-medicinally-medical-industry-algae-vessel-copper-turquoise-means-141103166.htmlRFJ5FPEP–blue medicinally medical industry algae vessel copper turquoise means agent
 Making copper sulphate crystals in a UK school science experiment Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/making-copper-sulphate-crystals-in-a-uk-school-science-experiment-image344146430.html
Making copper sulphate crystals in a UK school science experiment Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/making-copper-sulphate-crystals-in-a-uk-school-science-experiment-image344146430.htmlRF2AYW67X–Making copper sulphate crystals in a UK school science experiment
 full frame background and texture of blue copper sulfate granules - close-up. Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/full-frame-background-and-texture-of-blue-copper-sulfate-granules-close-up-image398515646.html
full frame background and texture of blue copper sulfate granules - close-up. Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/full-frame-background-and-texture-of-blue-copper-sulfate-granules-close-up-image398515646.htmlRF2E49XNJ–full frame background and texture of blue copper sulfate granules - close-up.
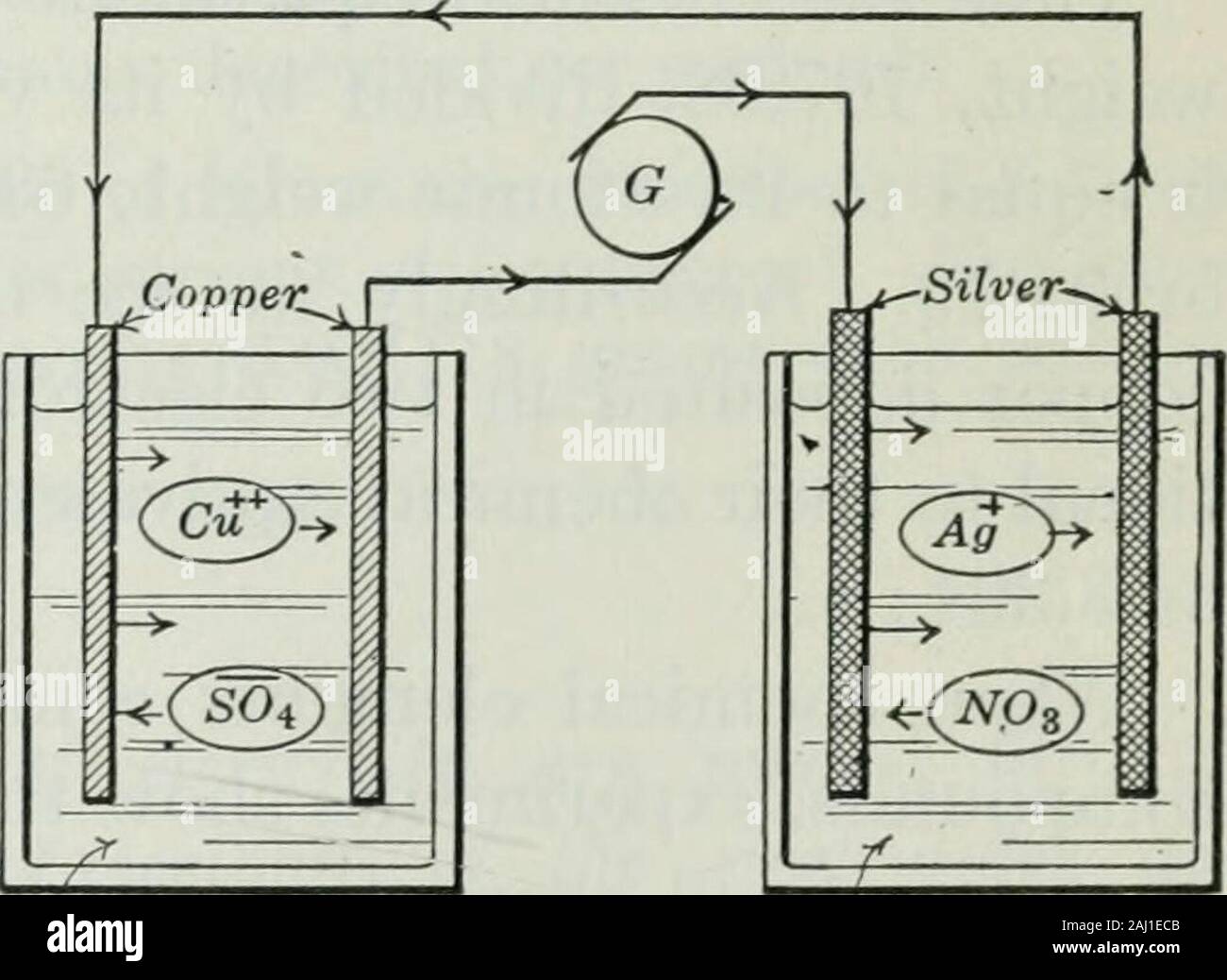 An elementary book on electricity and magnetism and their applications . up their negative chargesof electricity and react with the water (H20) to form sulphuricacid (H2SO4) and to set free oxygen (02). In this way thesulphuric acid, which is added to conduct the electricity, isnot used up, while the water (2 H20) is broken into hydrogen(2 H2) and oxygen (02). 180. Electrolytic cells in series. Suppose two electrolyticcells, one with copper plates in copper sulphate solution andthe other with silver platesin silver nitrate solution,are connected in series, asshown in figure 186, so thatthe sam Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/an-elementary-book-on-electricity-and-magnetism-and-their-applications-up-their-negative-chargesof-electricity-and-react-with-the-water-h20-to-form-sulphuricacid-h2so4-and-to-set-free-oxygen-02-in-this-way-thesulphuric-acid-which-is-added-to-conduct-the-electricity-isnot-used-up-while-the-water-2-h20-is-broken-into-hydrogen2-h2-and-oxygen-02-180-electrolytic-cells-in-series-suppose-two-electrolyticcells-one-with-copper-plates-in-copper-sulphate-solution-andthe-other-with-silver-platesin-silver-nitrate-solutionare-connected-in-series-asshown-in-figure-186-so-thatthe-sam-image338094075.html
An elementary book on electricity and magnetism and their applications . up their negative chargesof electricity and react with the water (H20) to form sulphuricacid (H2SO4) and to set free oxygen (02). In this way thesulphuric acid, which is added to conduct the electricity, isnot used up, while the water (2 H20) is broken into hydrogen(2 H2) and oxygen (02). 180. Electrolytic cells in series. Suppose two electrolyticcells, one with copper plates in copper sulphate solution andthe other with silver platesin silver nitrate solution,are connected in series, asshown in figure 186, so thatthe sam Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/an-elementary-book-on-electricity-and-magnetism-and-their-applications-up-their-negative-chargesof-electricity-and-react-with-the-water-h20-to-form-sulphuricacid-h2so4-and-to-set-free-oxygen-02-in-this-way-thesulphuric-acid-which-is-added-to-conduct-the-electricity-isnot-used-up-while-the-water-2-h20-is-broken-into-hydrogen2-h2-and-oxygen-02-180-electrolytic-cells-in-series-suppose-two-electrolyticcells-one-with-copper-plates-in-copper-sulphate-solution-andthe-other-with-silver-platesin-silver-nitrate-solutionare-connected-in-series-asshown-in-figure-186-so-thatthe-sam-image338094075.htmlRM2AJ1ECB–An elementary book on electricity and magnetism and their applications . up their negative chargesof electricity and react with the water (H20) to form sulphuricacid (H2SO4) and to set free oxygen (02). In this way thesulphuric acid, which is added to conduct the electricity, isnot used up, while the water (2 H20) is broken into hydrogen(2 H2) and oxygen (02). 180. Electrolytic cells in series. Suppose two electrolyticcells, one with copper plates in copper sulphate solution andthe other with silver platesin silver nitrate solution,are connected in series, asshown in figure 186, so thatthe sam
 full frame background and texture of blue copper sulfate granules - close-up Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/full-frame-background-and-texture-of-blue-copper-sulfate-granules-close-up-image464743713.html
full frame background and texture of blue copper sulfate granules - close-up Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/full-frame-background-and-texture-of-blue-copper-sulfate-granules-close-up-image464743713.htmlRF2J02W9N–full frame background and texture of blue copper sulfate granules - close-up
 Spraying an apple tree with fruits from codling moth and aphids. Treatment of apple trees with copper sulphate and ammonia. Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/spraying-an-apple-tree-with-fruits-from-codling-moth-and-aphids-treatment-of-apple-trees-with-copper-sulphate-and-ammonia-image556764436.html
Spraying an apple tree with fruits from codling moth and aphids. Treatment of apple trees with copper sulphate and ammonia. Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/spraying-an-apple-tree-with-fruits-from-codling-moth-and-aphids-treatment-of-apple-trees-with-copper-sulphate-and-ammonia-image556764436.htmlRF2R9PPM4–Spraying an apple tree with fruits from codling moth and aphids. Treatment of apple trees with copper sulphate and ammonia.
 an iron nail in copper sulphate solution at the start of plating Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/an-iron-nail-in-copper-sulphate-solution-at-the-start-of-plating-image1417660.html
an iron nail in copper sulphate solution at the start of plating Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/an-iron-nail-in-copper-sulphate-solution-at-the-start-of-plating-image1417660.htmlRMANA1BD–an iron nail in copper sulphate solution at the start of plating
 . Diseases of economic plants . Plant diseases. FUNGICIDES 27 gallon of this solution contains 2 pounds of copper sul- phate, and the necessity for further weighing is avoided. This solution will remain good for any length of time if the water evaporated is replaced. In order to dissolve the copper sulphate, suspend it in a coarse bag near the top of the water in a barrel. In this. Fig. 8. — A convenient arrangement for mixing Bordeaux mixture. After Vermont Agricultural Experiment Station. way it will dissolve in a few hours. If it is placed in the bottom of a barrel, it will dissolve but slo Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/diseases-of-economic-plants-plant-diseases-fungicides-27-gallon-of-this-solution-contains-2-pounds-of-copper-sul-phate-and-the-necessity-for-further-weighing-is-avoided-this-solution-will-remain-good-for-any-length-of-time-if-the-water-evaporated-is-replaced-in-order-to-dissolve-the-copper-sulphate-suspend-it-in-a-coarse-bag-near-the-top-of-the-water-in-a-barrel-in-this-fig-8-a-convenient-arrangement-for-mixing-bordeaux-mixture-after-vermont-agricultural-experiment-station-way-it-will-dissolve-in-a-few-hours-if-it-is-placed-in-the-bottom-of-a-barrel-it-will-dissolve-but-slo-image216458774.html
. Diseases of economic plants . Plant diseases. FUNGICIDES 27 gallon of this solution contains 2 pounds of copper sul- phate, and the necessity for further weighing is avoided. This solution will remain good for any length of time if the water evaporated is replaced. In order to dissolve the copper sulphate, suspend it in a coarse bag near the top of the water in a barrel. In this. Fig. 8. — A convenient arrangement for mixing Bordeaux mixture. After Vermont Agricultural Experiment Station. way it will dissolve in a few hours. If it is placed in the bottom of a barrel, it will dissolve but slo Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/diseases-of-economic-plants-plant-diseases-fungicides-27-gallon-of-this-solution-contains-2-pounds-of-copper-sul-phate-and-the-necessity-for-further-weighing-is-avoided-this-solution-will-remain-good-for-any-length-of-time-if-the-water-evaporated-is-replaced-in-order-to-dissolve-the-copper-sulphate-suspend-it-in-a-coarse-bag-near-the-top-of-the-water-in-a-barrel-in-this-fig-8-a-convenient-arrangement-for-mixing-bordeaux-mixture-after-vermont-agricultural-experiment-station-way-it-will-dissolve-in-a-few-hours-if-it-is-placed-in-the-bottom-of-a-barrel-it-will-dissolve-but-slo-image216458774.htmlRMPG4FAE–. Diseases of economic plants . Plant diseases. FUNGICIDES 27 gallon of this solution contains 2 pounds of copper sul- phate, and the necessity for further weighing is avoided. This solution will remain good for any length of time if the water evaporated is replaced. In order to dissolve the copper sulphate, suspend it in a coarse bag near the top of the water in a barrel. In this. Fig. 8. — A convenient arrangement for mixing Bordeaux mixture. After Vermont Agricultural Experiment Station. way it will dissolve in a few hours. If it is placed in the bottom of a barrel, it will dissolve but slo
 Petri dishes of copper sulphate solution isolated on white background Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/stock-image-petri-dishes-of-copper-sulphate-solution-isolated-on-white-background-162029301.html
Petri dishes of copper sulphate solution isolated on white background Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/stock-image-petri-dishes-of-copper-sulphate-solution-isolated-on-white-background-162029301.htmlRFKBH20N–Petri dishes of copper sulphate solution isolated on white background
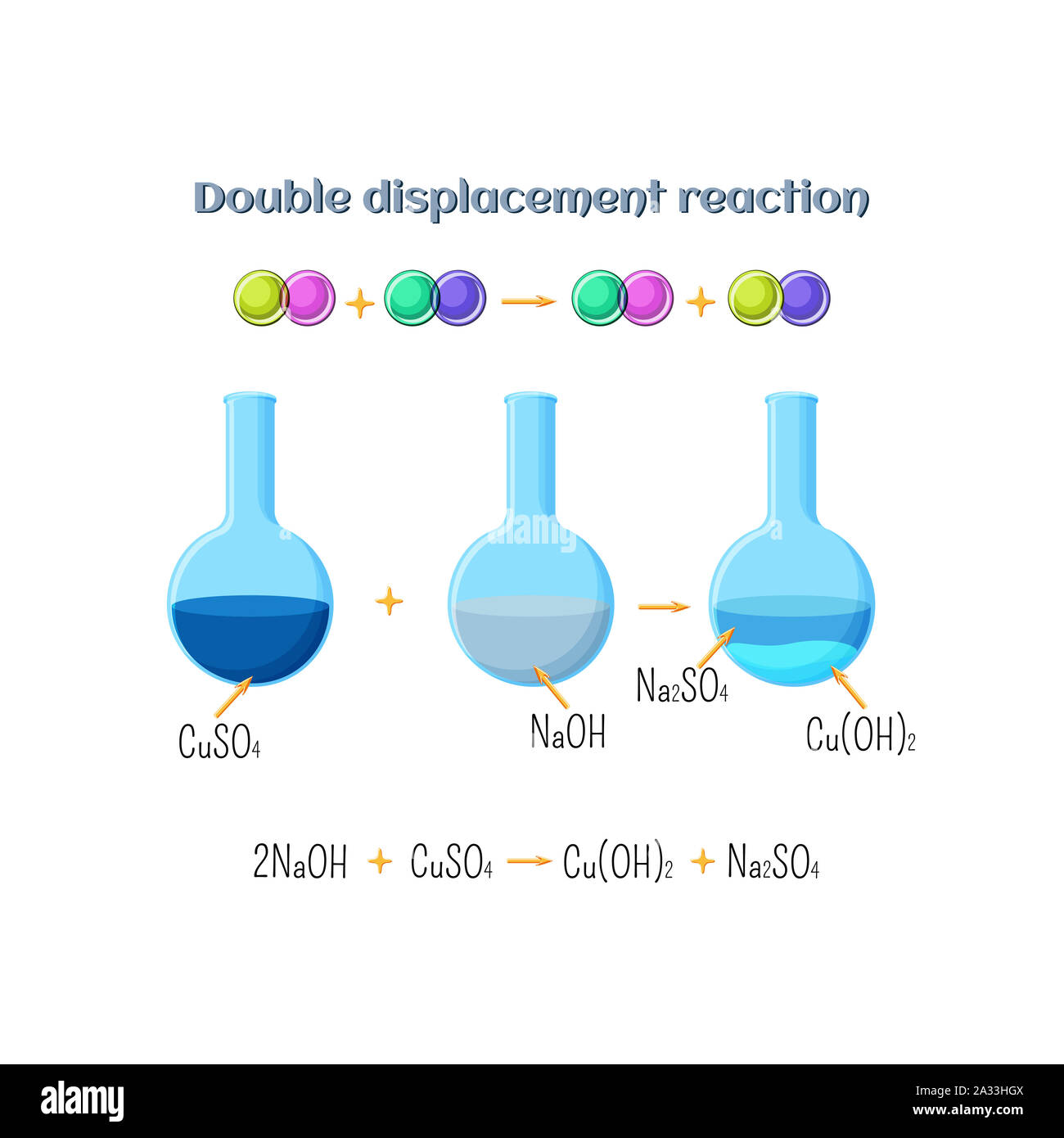 Double displacement reaction, illustration Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/double-displacement-reaction-illustration-image328920618.html
Double displacement reaction, illustration Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/double-displacement-reaction-illustration-image328920618.htmlRF2A33HGX–Double displacement reaction, illustration
 Glass ampoules containing iron supplement solution to treat anaemia. Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/glass-ampoules-containing-iron-supplement-solution-to-treat-anaemia-image470064340.html
Glass ampoules containing iron supplement solution to treat anaemia. Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/glass-ampoules-containing-iron-supplement-solution-to-treat-anaemia-image470064340.htmlRF2J8N7T4–Glass ampoules containing iron supplement solution to treat anaemia.
 bustion are termed supporters of combustion I face is composed—whether of rocks that rise Longmald's Improvement In Separating Me tals from their Ores. A very interesting paper was read before the London Society of Arts in their last meet ing in April last on Longmaid's Process for Separating Metals from their Ores. When common salt and minerals containing silver copper iron amd sulphur are mixed to gether and exposed to the combined action of heat and atmospheric air mutual decomposi tion ensues with formation of sulphate of soda and chloride of silver and copper soluble in the alkaline Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/bustion-are-termed-supporters-of-combustion-i-face-is-composedwhether-of-rocks-that-rise-longmalds-improvement-in-separating-me-tals-from-their-ores-a-very-interesting-paper-was-read-before-the-london-society-of-arts-in-their-last-meet-ing-in-april-last-on-longmaids-process-for-separating-metals-from-their-ores-when-common-salt-and-minerals-containing-silver-copper-iron-amd-sulphur-are-mixed-to-gether-and-exposed-to-the-combined-action-of-heat-and-atmospheric-air-mutual-decomposi-tion-ensues-with-formation-of-sulphate-of-soda-and-chloride-of-silver-and-copper-soluble-in-the-alkaline-image334300147.html
bustion are termed supporters of combustion I face is composed—whether of rocks that rise Longmald's Improvement In Separating Me tals from their Ores. A very interesting paper was read before the London Society of Arts in their last meet ing in April last on Longmaid's Process for Separating Metals from their Ores. When common salt and minerals containing silver copper iron amd sulphur are mixed to gether and exposed to the combined action of heat and atmospheric air mutual decomposi tion ensues with formation of sulphate of soda and chloride of silver and copper soluble in the alkaline Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/bustion-are-termed-supporters-of-combustion-i-face-is-composedwhether-of-rocks-that-rise-longmalds-improvement-in-separating-me-tals-from-their-ores-a-very-interesting-paper-was-read-before-the-london-society-of-arts-in-their-last-meet-ing-in-april-last-on-longmaids-process-for-separating-metals-from-their-ores-when-common-salt-and-minerals-containing-silver-copper-iron-amd-sulphur-are-mixed-to-gether-and-exposed-to-the-combined-action-of-heat-and-atmospheric-air-mutual-decomposi-tion-ensues-with-formation-of-sulphate-of-soda-and-chloride-of-silver-and-copper-soluble-in-the-alkaline-image334300147.htmlRM2ABTK6Y–bustion are termed supporters of combustion I face is composed—whether of rocks that rise Longmald's Improvement In Separating Me tals from their Ores. A very interesting paper was read before the London Society of Arts in their last meet ing in April last on Longmaid's Process for Separating Metals from their Ores. When common salt and minerals containing silver copper iron amd sulphur are mixed to gether and exposed to the combined action of heat and atmospheric air mutual decomposi tion ensues with formation of sulphate of soda and chloride of silver and copper soluble in the alkaline
 Eight lectures on the signs Eight lectures on the signs of life from their electrical aspect eightlecturesons00wall Year: 1903 FIG. 53-—Circular areas of skin of the forearm that have served as electrodes to a constant current for a few minutes. In the upper pair of circles the two electrodes in contact with the skin were a solution of permanganate of potash ; the coloured ion was the anion MnO4, which travels up stream and enters the body at the kathode. In the lower pair of circles, the two electrodes in contact with the skin were a solution of copper sulphate ; the coloured ion was the kat Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/eight-lectures-on-the-signs-eight-lectures-on-the-signs-of-life-from-their-electrical-aspect-eightlecturesons00wall-year-1903-fig-53-circular-areas-of-skin-of-the-forearm-that-have-served-as-electrodes-to-a-constant-current-for-a-few-minutes-in-the-upper-pair-of-circles-the-two-electrodes-in-contact-with-the-skin-were-a-solution-of-permanganate-of-potash-the-coloured-ion-was-the-anion-mno4-which-travels-up-stream-and-enters-the-body-at-the-kathode-in-the-lower-pair-of-circles-the-two-electrodes-in-contact-with-the-skin-were-a-solution-of-copper-sulphate-the-coloured-ion-was-the-kat-image239879892.html
Eight lectures on the signs Eight lectures on the signs of life from their electrical aspect eightlecturesons00wall Year: 1903 FIG. 53-—Circular areas of skin of the forearm that have served as electrodes to a constant current for a few minutes. In the upper pair of circles the two electrodes in contact with the skin were a solution of permanganate of potash ; the coloured ion was the anion MnO4, which travels up stream and enters the body at the kathode. In the lower pair of circles, the two electrodes in contact with the skin were a solution of copper sulphate ; the coloured ion was the kat Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/eight-lectures-on-the-signs-eight-lectures-on-the-signs-of-life-from-their-electrical-aspect-eightlecturesons00wall-year-1903-fig-53-circular-areas-of-skin-of-the-forearm-that-have-served-as-electrodes-to-a-constant-current-for-a-few-minutes-in-the-upper-pair-of-circles-the-two-electrodes-in-contact-with-the-skin-were-a-solution-of-permanganate-of-potash-the-coloured-ion-was-the-anion-mno4-which-travels-up-stream-and-enters-the-body-at-the-kathode-in-the-lower-pair-of-circles-the-two-electrodes-in-contact-with-the-skin-were-a-solution-of-copper-sulphate-the-coloured-ion-was-the-kat-image239879892.htmlRMRX7D70–Eight lectures on the signs Eight lectures on the signs of life from their electrical aspect eightlecturesons00wall Year: 1903 FIG. 53-—Circular areas of skin of the forearm that have served as electrodes to a constant current for a few minutes. In the upper pair of circles the two electrodes in contact with the skin were a solution of permanganate of potash ; the coloured ion was the anion MnO4, which travels up stream and enters the body at the kathode. In the lower pair of circles, the two electrodes in contact with the skin were a solution of copper sulphate ; the coloured ion was the kat
 . Fig. 23.—Early Stage of Foot-Rot. Fig. 24.—Advanced Foot-Rot. the sole, and gather the offending matter. When the disease occurs, carefully dress the sores with any prepared hoof ointment, after washing them in a solution of one pound of sulphate of cop- per in 5 gallons of water, and in the same proportion as one ounce to 1^ quarts of water. An excellent ointment for the feet so diseased is made in this way: Melt four parts of Burgundy pitch, add one part of vaseline, one part of turpentine, and one part of acetate of copper finely powdered, and stir until cool. Apply this to the pores. Kee Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/fig-23early-stage-of-foot-rot-fig-24advanced-foot-rot-the-sole-and-gather-the-offending-matter-when-the-disease-occurs-carefully-dress-the-sores-with-any-prepared-hoof-ointment-after-washing-them-in-a-solution-of-one-pound-of-sulphate-of-cop-per-in-5-gallons-of-water-and-in-the-same-proportion-as-one-ounce-to-1-quarts-of-water-an-excellent-ointment-for-the-feet-so-diseased-is-made-in-this-way-melt-four-parts-of-burgundy-pitch-add-one-part-of-vaseline-one-part-of-turpentine-and-one-part-of-acetate-of-copper-finely-powdered-and-stir-until-cool-apply-this-to-the-pores-kee-image179877498.html
. Fig. 23.—Early Stage of Foot-Rot. Fig. 24.—Advanced Foot-Rot. the sole, and gather the offending matter. When the disease occurs, carefully dress the sores with any prepared hoof ointment, after washing them in a solution of one pound of sulphate of cop- per in 5 gallons of water, and in the same proportion as one ounce to 1^ quarts of water. An excellent ointment for the feet so diseased is made in this way: Melt four parts of Burgundy pitch, add one part of vaseline, one part of turpentine, and one part of acetate of copper finely powdered, and stir until cool. Apply this to the pores. Kee Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/fig-23early-stage-of-foot-rot-fig-24advanced-foot-rot-the-sole-and-gather-the-offending-matter-when-the-disease-occurs-carefully-dress-the-sores-with-any-prepared-hoof-ointment-after-washing-them-in-a-solution-of-one-pound-of-sulphate-of-cop-per-in-5-gallons-of-water-and-in-the-same-proportion-as-one-ounce-to-1-quarts-of-water-an-excellent-ointment-for-the-feet-so-diseased-is-made-in-this-way-melt-four-parts-of-burgundy-pitch-add-one-part-of-vaseline-one-part-of-turpentine-and-one-part-of-acetate-of-copper-finely-powdered-and-stir-until-cool-apply-this-to-the-pores-kee-image179877498.htmlRMMCJ3GA–. Fig. 23.—Early Stage of Foot-Rot. Fig. 24.—Advanced Foot-Rot. the sole, and gather the offending matter. When the disease occurs, carefully dress the sores with any prepared hoof ointment, after washing them in a solution of one pound of sulphate of cop- per in 5 gallons of water, and in the same proportion as one ounce to 1^ quarts of water. An excellent ointment for the feet so diseased is made in this way: Melt four parts of Burgundy pitch, add one part of vaseline, one part of turpentine, and one part of acetate of copper finely powdered, and stir until cool. Apply this to the pores. Kee
 Various inorganic salts and Erlenmeyer flask with copper sulphate dissolved Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/stock-photo-various-inorganic-salts-and-erlenmeyer-flask-with-copper-sulphate-129898651.html
Various inorganic salts and Erlenmeyer flask with copper sulphate dissolved Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/stock-photo-various-inorganic-salts-and-erlenmeyer-flask-with-copper-sulphate-129898651.htmlRFHF9B1F–Various inorganic salts and Erlenmeyer flask with copper sulphate dissolved
 Cathodes put into electrolytic solution to get copper powder Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/cathodes-put-into-electrolytic-solution-to-get-copper-powder-image487833965.html
Cathodes put into electrolytic solution to get copper powder Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/cathodes-put-into-electrolytic-solution-to-get-copper-powder-image487833965.htmlRF2K9JN5H–Cathodes put into electrolytic solution to get copper powder
 Making copper sulphate crystals in a UK school science experiment Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/making-copper-sulphate-crystals-in-a-uk-school-science-experiment-image344146502.html
Making copper sulphate crystals in a UK school science experiment Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/making-copper-sulphate-crystals-in-a-uk-school-science-experiment-image344146502.htmlRF2AYW6AE–Making copper sulphate crystals in a UK school science experiment
 Making copper sulphate crystals in a UK school science experiment Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/making-copper-sulphate-crystals-in-a-uk-school-science-experiment-image344146500.html
Making copper sulphate crystals in a UK school science experiment Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/making-copper-sulphate-crystals-in-a-uk-school-science-experiment-image344146500.htmlRF2AYW6AC–Making copper sulphate crystals in a UK school science experiment
 . Practical electricity in medicine and surgery. Fig. SO. Fiu. 87. single fluid by two fluids. In these batteries two solutions areemployed, and they are separated from each other either bytaking advantage of their different specific gravities or by meansof porous earthenware vessels. Daniell Cell.—Probably the best-known type of the two-fluid cell is that called the Daniell Element (Fig. 86). In thiscell the fluids are a saturated solution of sulphate of copper,CS, and a semi-saturated solution of sulphate of zinc, ZS.In the copper-sulphate solution is placed a sheet of metalliccopper, C, and Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/practical-electricity-in-medicine-and-surgery-fig-so-fiu-87-single-fluid-by-two-fluids-in-these-batteries-two-solutions-areemployed-and-they-are-separated-from-each-other-either-bytaking-advantage-of-their-different-specific-gravities-or-by-meansof-porous-earthenware-vessels-daniell-cellprobably-the-best-known-type-of-the-two-fluid-cell-is-that-called-the-daniell-element-fig-86-in-thiscell-the-fluids-are-a-saturated-solution-of-sulphate-of-coppercs-and-a-semi-saturated-solution-of-sulphate-of-zinc-zsin-the-copper-sulphate-solution-is-placed-a-sheet-of-metalliccopper-c-and-image336698053.html
. Practical electricity in medicine and surgery. Fig. SO. Fiu. 87. single fluid by two fluids. In these batteries two solutions areemployed, and they are separated from each other either bytaking advantage of their different specific gravities or by meansof porous earthenware vessels. Daniell Cell.—Probably the best-known type of the two-fluid cell is that called the Daniell Element (Fig. 86). In thiscell the fluids are a saturated solution of sulphate of copper,CS, and a semi-saturated solution of sulphate of zinc, ZS.In the copper-sulphate solution is placed a sheet of metalliccopper, C, and Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/practical-electricity-in-medicine-and-surgery-fig-so-fiu-87-single-fluid-by-two-fluids-in-these-batteries-two-solutions-areemployed-and-they-are-separated-from-each-other-either-bytaking-advantage-of-their-different-specific-gravities-or-by-meansof-porous-earthenware-vessels-daniell-cellprobably-the-best-known-type-of-the-two-fluid-cell-is-that-called-the-daniell-element-fig-86-in-thiscell-the-fluids-are-a-saturated-solution-of-sulphate-of-coppercs-and-a-semi-saturated-solution-of-sulphate-of-zinc-zsin-the-copper-sulphate-solution-is-placed-a-sheet-of-metalliccopper-c-and-image336698053.htmlRM2AFNWPD–. Practical electricity in medicine and surgery. Fig. SO. Fiu. 87. single fluid by two fluids. In these batteries two solutions areemployed, and they are separated from each other either bytaking advantage of their different specific gravities or by meansof porous earthenware vessels. Daniell Cell.—Probably the best-known type of the two-fluid cell is that called the Daniell Element (Fig. 86). In thiscell the fluids are a saturated solution of sulphate of copper,CS, and a semi-saturated solution of sulphate of zinc, ZS.In the copper-sulphate solution is placed a sheet of metalliccopper, C, and
 full frame background and texture of blue copper sulfate granules - close-up Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/full-frame-background-and-texture-of-blue-copper-sulfate-granules-close-up-image464691779.html
full frame background and texture of blue copper sulfate granules - close-up Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/full-frame-background-and-texture-of-blue-copper-sulfate-granules-close-up-image464691779.htmlRF2J00F2Y–full frame background and texture of blue copper sulfate granules - close-up
 copper plated nail being removed from copper sulphate solution Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/copper-plated-nail-being-removed-from-copper-sulphate-solution-image1417655.html
copper plated nail being removed from copper sulphate solution Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/copper-plated-nail-being-removed-from-copper-sulphate-solution-image1417655.htmlRMANA1B8–copper plated nail being removed from copper sulphate solution
 . The American fruit culturist, containing directions for the propagation and culture of all fruits adapted to the United States. Fruit-culture. 234 THE DISEASES OF FRUITS. the winter the vines and trellises may well be treated with a wash of copper sulphate, one pound to thirty gallons of water. This use of the plain bluestone solution is of special value as a cleansing solution, and can be safely employed upon stems not leaf-bearing at the time. Last, but not least, is the use of Bordeaux upon the vines during the growing season, with cupram as the fruit nears maturity. In this way there is Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/the-american-fruit-culturist-containing-directions-for-the-propagation-and-culture-of-all-fruits-adapted-to-the-united-states-fruit-culture-234-the-diseases-of-fruits-the-winter-the-vines-and-trellises-may-well-be-treated-with-a-wash-of-copper-sulphate-one-pound-to-thirty-gallons-of-water-this-use-of-the-plain-bluestone-solution-is-of-special-value-as-a-cleansing-solution-and-can-be-safely-employed-upon-stems-not-leaf-bearing-at-the-time-last-but-not-least-is-the-use-of-bordeaux-upon-the-vines-during-the-growing-season-with-cupram-as-the-fruit-nears-maturity-in-this-way-there-is-image216359603.html
. The American fruit culturist, containing directions for the propagation and culture of all fruits adapted to the United States. Fruit-culture. 234 THE DISEASES OF FRUITS. the winter the vines and trellises may well be treated with a wash of copper sulphate, one pound to thirty gallons of water. This use of the plain bluestone solution is of special value as a cleansing solution, and can be safely employed upon stems not leaf-bearing at the time. Last, but not least, is the use of Bordeaux upon the vines during the growing season, with cupram as the fruit nears maturity. In this way there is Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/the-american-fruit-culturist-containing-directions-for-the-propagation-and-culture-of-all-fruits-adapted-to-the-united-states-fruit-culture-234-the-diseases-of-fruits-the-winter-the-vines-and-trellises-may-well-be-treated-with-a-wash-of-copper-sulphate-one-pound-to-thirty-gallons-of-water-this-use-of-the-plain-bluestone-solution-is-of-special-value-as-a-cleansing-solution-and-can-be-safely-employed-upon-stems-not-leaf-bearing-at-the-time-last-but-not-least-is-the-use-of-bordeaux-upon-the-vines-during-the-growing-season-with-cupram-as-the-fruit-nears-maturity-in-this-way-there-is-image216359603.htmlRMPG00TK–. The American fruit culturist, containing directions for the propagation and culture of all fruits adapted to the United States. Fruit-culture. 234 THE DISEASES OF FRUITS. the winter the vines and trellises may well be treated with a wash of copper sulphate, one pound to thirty gallons of water. This use of the plain bluestone solution is of special value as a cleansing solution, and can be safely employed upon stems not leaf-bearing at the time. Last, but not least, is the use of Bordeaux upon the vines during the growing season, with cupram as the fruit nears maturity. In this way there is
 Petri dishes of copper sulphate solution isolated on white background Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/stock-image-petri-dishes-of-copper-sulphate-solution-isolated-on-white-background-162029334.html
Petri dishes of copper sulphate solution isolated on white background Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/stock-image-petri-dishes-of-copper-sulphate-solution-isolated-on-white-background-162029334.htmlRFKBH21X–Petri dishes of copper sulphate solution isolated on white background
 Precipitation reaction, illustration Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/precipitation-reaction-illustration-image328920507.html
Precipitation reaction, illustration Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/precipitation-reaction-illustration-image328920507.htmlRF2A33HCY–Precipitation reaction, illustration
 Glass ampoules containing iron supplement solution to treat anaemia. Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/glass-ampoules-containing-iron-supplement-solution-to-treat-anaemia-image470064675.html
Glass ampoules containing iron supplement solution to treat anaemia. Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/glass-ampoules-containing-iron-supplement-solution-to-treat-anaemia-image470064675.htmlRF2J8N883–Glass ampoules containing iron supplement solution to treat anaemia.
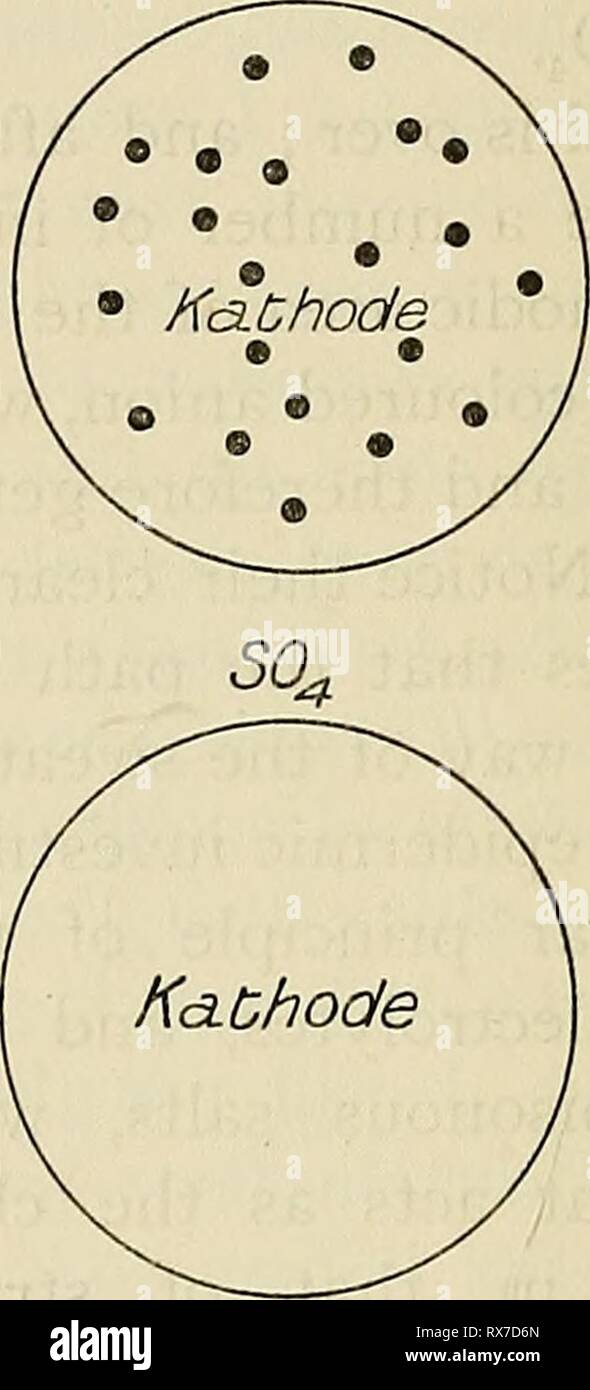 Eight lectures on the signs Eight lectures on the signs of life from their electrical aspect eightlectureson00wall Year: 1903 Fig. 53.—Circular areae of skin of the forearm that have served as electrodes to a constant current for a few minutes. In the upper pair of circles the two electrodes in contact with the skin were a solution of permanganate of potash ; the coloured ion was the anion Mn04, which travels up stream and enters the body at the kathode. In the lower pair of circles, the two electrodes in contact with the skin were a solution of copper sulphate ; the coloured ion was the kati Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/eight-lectures-on-the-signs-eight-lectures-on-the-signs-of-life-from-their-electrical-aspect-eightlectureson00wall-year-1903-fig-53circular-areae-of-skin-of-the-forearm-that-have-served-as-electrodes-to-a-constant-current-for-a-few-minutes-in-the-upper-pair-of-circles-the-two-electrodes-in-contact-with-the-skin-were-a-solution-of-permanganate-of-potash-the-coloured-ion-was-the-anion-mn04-which-travels-up-stream-and-enters-the-body-at-the-kathode-in-the-lower-pair-of-circles-the-two-electrodes-in-contact-with-the-skin-were-a-solution-of-copper-sulphate-the-coloured-ion-was-the-kati-image239879885.html
Eight lectures on the signs Eight lectures on the signs of life from their electrical aspect eightlectureson00wall Year: 1903 Fig. 53.—Circular areae of skin of the forearm that have served as electrodes to a constant current for a few minutes. In the upper pair of circles the two electrodes in contact with the skin were a solution of permanganate of potash ; the coloured ion was the anion Mn04, which travels up stream and enters the body at the kathode. In the lower pair of circles, the two electrodes in contact with the skin were a solution of copper sulphate ; the coloured ion was the kati Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/eight-lectures-on-the-signs-eight-lectures-on-the-signs-of-life-from-their-electrical-aspect-eightlectureson00wall-year-1903-fig-53circular-areae-of-skin-of-the-forearm-that-have-served-as-electrodes-to-a-constant-current-for-a-few-minutes-in-the-upper-pair-of-circles-the-two-electrodes-in-contact-with-the-skin-were-a-solution-of-permanganate-of-potash-the-coloured-ion-was-the-anion-mn04-which-travels-up-stream-and-enters-the-body-at-the-kathode-in-the-lower-pair-of-circles-the-two-electrodes-in-contact-with-the-skin-were-a-solution-of-copper-sulphate-the-coloured-ion-was-the-kati-image239879885.htmlRMRX7D6N–Eight lectures on the signs Eight lectures on the signs of life from their electrical aspect eightlectureson00wall Year: 1903 Fig. 53.—Circular areae of skin of the forearm that have served as electrodes to a constant current for a few minutes. In the upper pair of circles the two electrodes in contact with the skin were a solution of permanganate of potash ; the coloured ion was the anion Mn04, which travels up stream and enters the body at the kathode. In the lower pair of circles, the two electrodes in contact with the skin were a solution of copper sulphate ; the coloured ion was the kati
 . Eight lectures on the signs of life from their electrical aspect . Fig. 53.—Circular areae of skin of the forearm that have served as electrodes to a constant current for a few minutes. In the upper pair of circles the two electrodes in contact with the skin were a solution of permanganate of potash ; the coloured ion was the anion Mn04, which travels up stream and enters the body at the kathode. In the lower pair of circles, the two electrodes in contact with the skin were a solution of copper sulphate ; the coloured ion was the kation Cu, which travels down stream and enters the body at th Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/eight-lectures-on-the-signs-of-life-from-their-electrical-aspect-fig-53circular-areae-of-skin-of-the-forearm-that-have-served-as-electrodes-to-a-constant-current-for-a-few-minutes-in-the-upper-pair-of-circles-the-two-electrodes-in-contact-with-the-skin-were-a-solution-of-permanganate-of-potash-the-coloured-ion-was-the-anion-mn04-which-travels-up-stream-and-enters-the-body-at-the-kathode-in-the-lower-pair-of-circles-the-two-electrodes-in-contact-with-the-skin-were-a-solution-of-copper-sulphate-the-coloured-ion-was-the-kation-cu-which-travels-down-stream-and-enters-the-body-at-th-image178416784.html
. Eight lectures on the signs of life from their electrical aspect . Fig. 53.—Circular areae of skin of the forearm that have served as electrodes to a constant current for a few minutes. In the upper pair of circles the two electrodes in contact with the skin were a solution of permanganate of potash ; the coloured ion was the anion Mn04, which travels up stream and enters the body at the kathode. In the lower pair of circles, the two electrodes in contact with the skin were a solution of copper sulphate ; the coloured ion was the kation Cu, which travels down stream and enters the body at th Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/eight-lectures-on-the-signs-of-life-from-their-electrical-aspect-fig-53circular-areae-of-skin-of-the-forearm-that-have-served-as-electrodes-to-a-constant-current-for-a-few-minutes-in-the-upper-pair-of-circles-the-two-electrodes-in-contact-with-the-skin-were-a-solution-of-permanganate-of-potash-the-coloured-ion-was-the-anion-mn04-which-travels-up-stream-and-enters-the-body-at-the-kathode-in-the-lower-pair-of-circles-the-two-electrodes-in-contact-with-the-skin-were-a-solution-of-copper-sulphate-the-coloured-ion-was-the-kation-cu-which-travels-down-stream-and-enters-the-body-at-th-image178416784.htmlRMMA7GC0–. Eight lectures on the signs of life from their electrical aspect . Fig. 53.—Circular areae of skin of the forearm that have served as electrodes to a constant current for a few minutes. In the upper pair of circles the two electrodes in contact with the skin were a solution of permanganate of potash ; the coloured ion was the anion Mn04, which travels up stream and enters the body at the kathode. In the lower pair of circles, the two electrodes in contact with the skin were a solution of copper sulphate ; the coloured ion was the kation Cu, which travels down stream and enters the body at th
 Chemical glassware on white background Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/stock-photo-chemical-glassware-on-white-background-129898652.html
Chemical glassware on white background Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/stock-photo-chemical-glassware-on-white-background-129898652.htmlRMHF9B1G–Chemical glassware on white background
 Cathodes put into electrolytic solution to get copper powder Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/cathodes-put-into-electrolytic-solution-to-get-copper-powder-image472541703.html
Cathodes put into electrolytic solution to get copper powder Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/cathodes-put-into-electrolytic-solution-to-get-copper-powder-image472541703.htmlRF2JCP3NB–Cathodes put into electrolytic solution to get copper powder
 Measuring cylinder, volumetric flask and protective glasses Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/stock-photo-measuring-cylinder-volumetric-flask-and-protective-glasses-92264141.html
Measuring cylinder, volumetric flask and protective glasses Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/stock-photo-measuring-cylinder-volumetric-flask-and-protective-glasses-92264141.htmlRMFA2YRW–Measuring cylinder, volumetric flask and protective glasses
 Making copper sulphate crystals in a UK school science experiment Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/making-copper-sulphate-crystals-in-a-uk-school-science-experiment-image344146550.html
Making copper sulphate crystals in a UK school science experiment Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/making-copper-sulphate-crystals-in-a-uk-school-science-experiment-image344146550.htmlRF2AYW6C6–Making copper sulphate crystals in a UK school science experiment
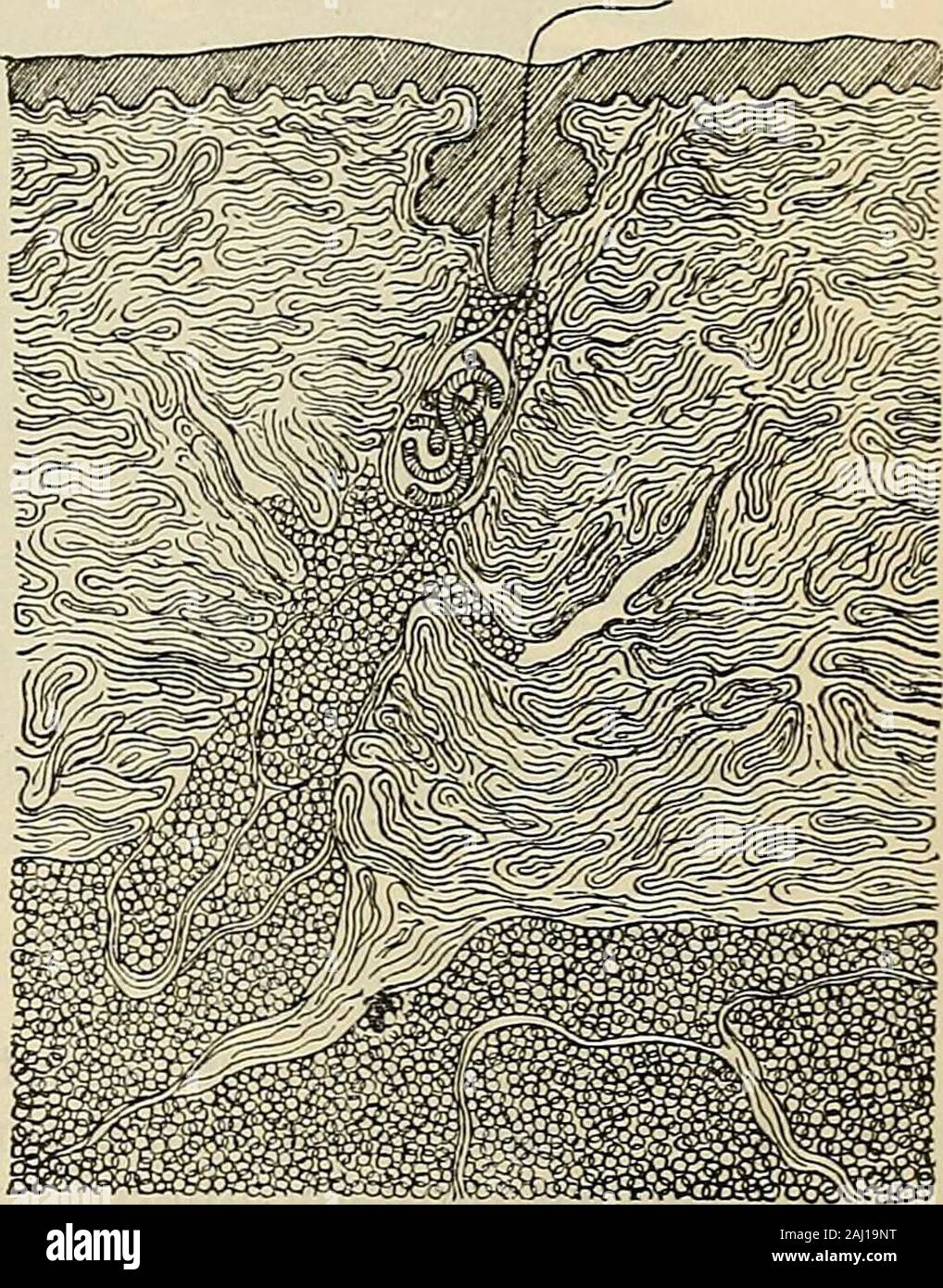 Modern surgery, general and operative . ize for at least a year, and leave ineradicable scars.This condition is due to a protozoon. Man is infected by means of flies,lice, or other insects. The Aleppo boil was once apparently confined to India,Arabia, Persia, Egypt, Algeria,- etc. Of late it is said to have appeared inPanama, the Philippine Islands, and Hawaii. Treatment.—Excision of ulcers has been advocated; so has cauterization.A useful treatment is to dress the areas with copper sulphate solution, graduallyincreasing the strength from i per cent, to 5 per cent. Carbuncle {benign anthrax) i Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/modern-surgery-general-and-operative-ize-for-at-least-a-year-and-leave-ineradicable-scarsthis-condition-is-due-to-a-protozoon-man-is-infected-by-means-of-flieslice-or-other-insects-the-aleppo-boil-was-once-apparently-confined-to-indiaarabia-persia-egypt-algeria-etc-of-late-it-is-said-to-have-appeared-inpanama-the-philippine-islands-and-hawaii-treatmentexcision-of-ulcers-has-been-advocated-so-has-cauterizationa-useful-treatment-is-to-dress-the-areas-with-copper-sulphate-solution-graduallyincreasing-the-strength-from-i-per-cent-to-5-per-cent-carbuncle-benign-anthrax-i-image338090420.html
Modern surgery, general and operative . ize for at least a year, and leave ineradicable scars.This condition is due to a protozoon. Man is infected by means of flies,lice, or other insects. The Aleppo boil was once apparently confined to India,Arabia, Persia, Egypt, Algeria,- etc. Of late it is said to have appeared inPanama, the Philippine Islands, and Hawaii. Treatment.—Excision of ulcers has been advocated; so has cauterization.A useful treatment is to dress the areas with copper sulphate solution, graduallyincreasing the strength from i per cent, to 5 per cent. Carbuncle {benign anthrax) i Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/modern-surgery-general-and-operative-ize-for-at-least-a-year-and-leave-ineradicable-scarsthis-condition-is-due-to-a-protozoon-man-is-infected-by-means-of-flieslice-or-other-insects-the-aleppo-boil-was-once-apparently-confined-to-indiaarabia-persia-egypt-algeria-etc-of-late-it-is-said-to-have-appeared-inpanama-the-philippine-islands-and-hawaii-treatmentexcision-of-ulcers-has-been-advocated-so-has-cauterizationa-useful-treatment-is-to-dress-the-areas-with-copper-sulphate-solution-graduallyincreasing-the-strength-from-i-per-cent-to-5-per-cent-carbuncle-benign-anthrax-i-image338090420.htmlRM2AJ19NT–Modern surgery, general and operative . ize for at least a year, and leave ineradicable scars.This condition is due to a protozoon. Man is infected by means of flies,lice, or other insects. The Aleppo boil was once apparently confined to India,Arabia, Persia, Egypt, Algeria,- etc. Of late it is said to have appeared inPanama, the Philippine Islands, and Hawaii. Treatment.—Excision of ulcers has been advocated; so has cauterization.A useful treatment is to dress the areas with copper sulphate solution, graduallyincreasing the strength from i per cent, to 5 per cent. Carbuncle {benign anthrax) i
 Making copper sulphate crystals in a UK school science experiment Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/making-copper-sulphate-crystals-in-a-uk-school-science-experiment-image344146501.html
Making copper sulphate crystals in a UK school science experiment Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/making-copper-sulphate-crystals-in-a-uk-school-science-experiment-image344146501.htmlRF2AYW6AD–Making copper sulphate crystals in a UK school science experiment
 full frame background and texture of blue copper sulfate granules - close-up Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/full-frame-background-and-texture-of-blue-copper-sulfate-granules-close-up-image464764756.html
full frame background and texture of blue copper sulfate granules - close-up Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/full-frame-background-and-texture-of-blue-copper-sulfate-granules-close-up-image464764756.htmlRF2J03T58–full frame background and texture of blue copper sulfate granules - close-up
 an iron nail after being left in copper sulphate solution Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/an-iron-nail-after-being-left-in-copper-sulphate-solution-image1417657.html
an iron nail after being left in copper sulphate solution Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/an-iron-nail-after-being-left-in-copper-sulphate-solution-image1417657.htmlRMANA1BA–an iron nail after being left in copper sulphate solution
 . Beginners' guide to fruit growing : a simple statement of the elementary practices of propagation, planting, culture, fertilization, pruning, spraying, etc. Fruit-culture. SPRAYING n Spraying are apple scab, apple tree canker, peach leaf curl and black knot of plum. BORDEAUX MIXTURE The standard solution for the prevention of fungous attacks is the bordeaux mixture composed of lime and copper sulphate. This is the great and ,^..i'Z>^. i FIG. 39—SPRING CAMPAIGN AGAINST SAN JOSE SCALE original spraying solution, and the one still most widely used. It is effective in the prevention of most p Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/beginners-guide-to-fruit-growing-a-simple-statement-of-the-elementary-practices-of-propagation-planting-culture-fertilization-pruning-spraying-etc-fruit-culture-spraying-n-spraying-are-apple-scab-apple-tree-canker-peach-leaf-curl-and-black-knot-of-plum-bordeaux-mixture-the-standard-solution-for-the-prevention-of-fungous-attacks-is-the-bordeaux-mixture-composed-of-lime-and-copper-sulphate-this-is-the-great-and-izgt-i-fig-39spring-campaign-against-san-jose-scale-original-spraying-solution-and-the-one-still-most-widely-used-it-is-effective-in-the-prevention-of-most-p-image216458616.html
. Beginners' guide to fruit growing : a simple statement of the elementary practices of propagation, planting, culture, fertilization, pruning, spraying, etc. Fruit-culture. SPRAYING n Spraying are apple scab, apple tree canker, peach leaf curl and black knot of plum. BORDEAUX MIXTURE The standard solution for the prevention of fungous attacks is the bordeaux mixture composed of lime and copper sulphate. This is the great and ,^..i'Z>^. i FIG. 39—SPRING CAMPAIGN AGAINST SAN JOSE SCALE original spraying solution, and the one still most widely used. It is effective in the prevention of most p Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/beginners-guide-to-fruit-growing-a-simple-statement-of-the-elementary-practices-of-propagation-planting-culture-fertilization-pruning-spraying-etc-fruit-culture-spraying-n-spraying-are-apple-scab-apple-tree-canker-peach-leaf-curl-and-black-knot-of-plum-bordeaux-mixture-the-standard-solution-for-the-prevention-of-fungous-attacks-is-the-bordeaux-mixture-composed-of-lime-and-copper-sulphate-this-is-the-great-and-izgt-i-fig-39spring-campaign-against-san-jose-scale-original-spraying-solution-and-the-one-still-most-widely-used-it-is-effective-in-the-prevention-of-most-p-image216458616.htmlRMPG4F4T–. Beginners' guide to fruit growing : a simple statement of the elementary practices of propagation, planting, culture, fertilization, pruning, spraying, etc. Fruit-culture. SPRAYING n Spraying are apple scab, apple tree canker, peach leaf curl and black knot of plum. BORDEAUX MIXTURE The standard solution for the prevention of fungous attacks is the bordeaux mixture composed of lime and copper sulphate. This is the great and ,^..i'Z>^. i FIG. 39—SPRING CAMPAIGN AGAINST SAN JOSE SCALE original spraying solution, and the one still most widely used. It is effective in the prevention of most p
 Petri dishes of copper sulphate solution isolated on white background Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/stock-image-petri-dishes-of-copper-sulphate-solution-isolated-on-white-background-162029292.html
Petri dishes of copper sulphate solution isolated on white background Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/stock-image-petri-dishes-of-copper-sulphate-solution-isolated-on-white-background-162029292.htmlRFKBH20C–Petri dishes of copper sulphate solution isolated on white background
 Glass ampoules containing iron supplement solution to treat anaemia. Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/glass-ampoules-containing-iron-supplement-solution-to-treat-anaemia-image470064742.html
Glass ampoules containing iron supplement solution to treat anaemia. Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/glass-ampoules-containing-iron-supplement-solution-to-treat-anaemia-image470064742.htmlRF2J8N8AE–Glass ampoules containing iron supplement solution to treat anaemia.
 Eight lectures on the signs Eight lectures on the signs of life from their electrical aspect . eightlectureson00wall Year: 1903 Fig. 53.—Circular areae of skin of the forearm that have served as electrodes to a constant current for a few minutes. In the upper pair of circles the two electrodes in contact with the skin were a solution of permanganate of potash ; the coloured ion was the anion Mn04, which travels up stream and enters the body at the kathode. In the lower pair of circles, the two electrodes in contact with the skin were a solution of copper sulphate ; the coloured ion was the ka Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/eight-lectures-on-the-signs-eight-lectures-on-the-signs-of-life-from-their-electrical-aspect-eightlectureson00wall-year-1903-fig-53circular-areae-of-skin-of-the-forearm-that-have-served-as-electrodes-to-a-constant-current-for-a-few-minutes-in-the-upper-pair-of-circles-the-two-electrodes-in-contact-with-the-skin-were-a-solution-of-permanganate-of-potash-the-coloured-ion-was-the-anion-mn04-which-travels-up-stream-and-enters-the-body-at-the-kathode-in-the-lower-pair-of-circles-the-two-electrodes-in-contact-with-the-skin-were-a-solution-of-copper-sulphate-the-coloured-ion-was-the-ka-image240859408.html
Eight lectures on the signs Eight lectures on the signs of life from their electrical aspect . eightlectureson00wall Year: 1903 Fig. 53.—Circular areae of skin of the forearm that have served as electrodes to a constant current for a few minutes. In the upper pair of circles the two electrodes in contact with the skin were a solution of permanganate of potash ; the coloured ion was the anion Mn04, which travels up stream and enters the body at the kathode. In the lower pair of circles, the two electrodes in contact with the skin were a solution of copper sulphate ; the coloured ion was the ka Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/eight-lectures-on-the-signs-eight-lectures-on-the-signs-of-life-from-their-electrical-aspect-eightlectureson00wall-year-1903-fig-53circular-areae-of-skin-of-the-forearm-that-have-served-as-electrodes-to-a-constant-current-for-a-few-minutes-in-the-upper-pair-of-circles-the-two-electrodes-in-contact-with-the-skin-were-a-solution-of-permanganate-of-potash-the-coloured-ion-was-the-anion-mn04-which-travels-up-stream-and-enters-the-body-at-the-kathode-in-the-lower-pair-of-circles-the-two-electrodes-in-contact-with-the-skin-were-a-solution-of-copper-sulphate-the-coloured-ion-was-the-ka-image240859408.htmlRMRYT2HM–Eight lectures on the signs Eight lectures on the signs of life from their electrical aspect . eightlectureson00wall Year: 1903 Fig. 53.—Circular areae of skin of the forearm that have served as electrodes to a constant current for a few minutes. In the upper pair of circles the two electrodes in contact with the skin were a solution of permanganate of potash ; the coloured ion was the anion Mn04, which travels up stream and enters the body at the kathode. In the lower pair of circles, the two electrodes in contact with the skin were a solution of copper sulphate ; the coloured ion was the ka
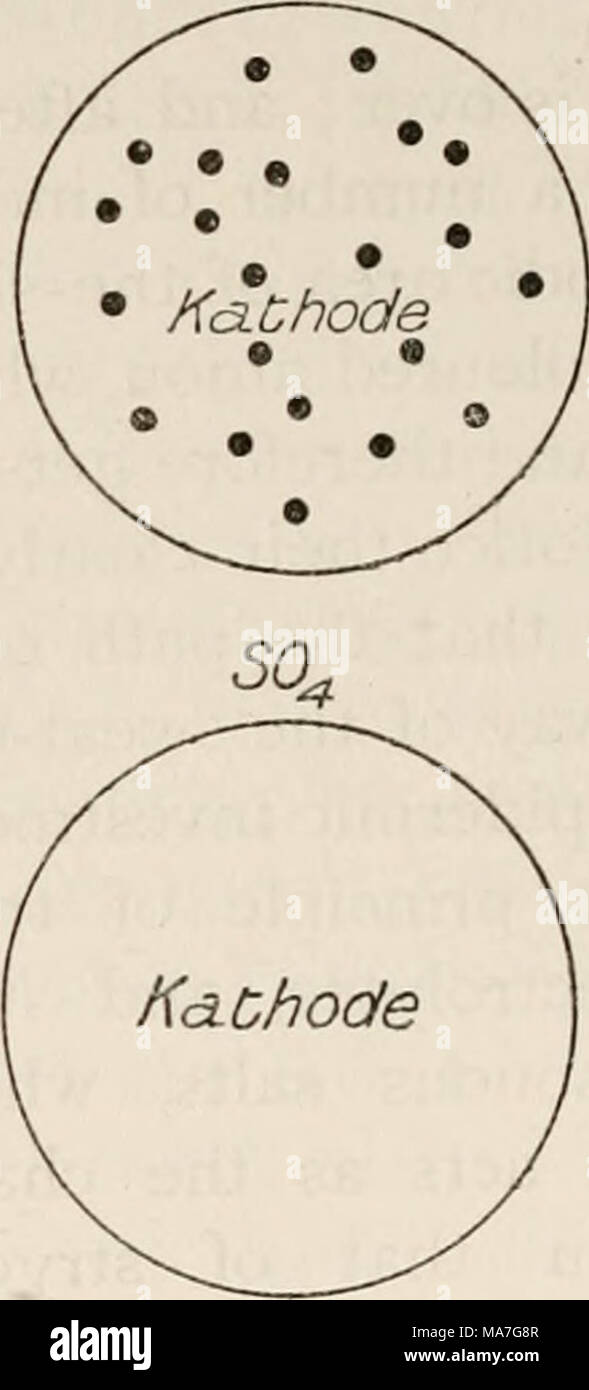 . Eight lectures on the signs of life from their electrical aspect . FIG. 53-—Circular areas of skin of the forearm that have served as electrodes to a constant current for a few minutes. In the upper pair of circles the two electrodes in contact with the skin were a solution of permanganate of potash ; the coloured ion was the anion MnO4, which travels up stream and enters the body at the kathode. In the lower pair of circles, the two electrodes in contact with the skin were a solution of copper sulphate ; the coloured ion was the kation Cu, which travels down stream and enters the body at th Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/eight-lectures-on-the-signs-of-life-from-their-electrical-aspect-fig-53-circular-areas-of-skin-of-the-forearm-that-have-served-as-electrodes-to-a-constant-current-for-a-few-minutes-in-the-upper-pair-of-circles-the-two-electrodes-in-contact-with-the-skin-were-a-solution-of-permanganate-of-potash-the-coloured-ion-was-the-anion-mno4-which-travels-up-stream-and-enters-the-body-at-the-kathode-in-the-lower-pair-of-circles-the-two-electrodes-in-contact-with-the-skin-were-a-solution-of-copper-sulphate-the-coloured-ion-was-the-kation-cu-which-travels-down-stream-and-enters-the-body-at-th-image178416695.html
. Eight lectures on the signs of life from their electrical aspect . FIG. 53-—Circular areas of skin of the forearm that have served as electrodes to a constant current for a few minutes. In the upper pair of circles the two electrodes in contact with the skin were a solution of permanganate of potash ; the coloured ion was the anion MnO4, which travels up stream and enters the body at the kathode. In the lower pair of circles, the two electrodes in contact with the skin were a solution of copper sulphate ; the coloured ion was the kation Cu, which travels down stream and enters the body at th Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/eight-lectures-on-the-signs-of-life-from-their-electrical-aspect-fig-53-circular-areas-of-skin-of-the-forearm-that-have-served-as-electrodes-to-a-constant-current-for-a-few-minutes-in-the-upper-pair-of-circles-the-two-electrodes-in-contact-with-the-skin-were-a-solution-of-permanganate-of-potash-the-coloured-ion-was-the-anion-mno4-which-travels-up-stream-and-enters-the-body-at-the-kathode-in-the-lower-pair-of-circles-the-two-electrodes-in-contact-with-the-skin-were-a-solution-of-copper-sulphate-the-coloured-ion-was-the-kation-cu-which-travels-down-stream-and-enters-the-body-at-th-image178416695.htmlRMMA7G8R–. Eight lectures on the signs of life from their electrical aspect . FIG. 53-—Circular areas of skin of the forearm that have served as electrodes to a constant current for a few minutes. In the upper pair of circles the two electrodes in contact with the skin were a solution of permanganate of potash ; the coloured ion was the anion MnO4, which travels up stream and enters the body at the kathode. In the lower pair of circles, the two electrodes in contact with the skin were a solution of copper sulphate ; the coloured ion was the kation Cu, which travels down stream and enters the body at th
 Cathodes put into electrolytic solution to get copper powder Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/cathodes-put-into-electrolytic-solution-to-get-copper-powder-image472315722.html
Cathodes put into electrolytic solution to get copper powder Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/cathodes-put-into-electrolytic-solution-to-get-copper-powder-image472315722.htmlRF2JCBREJ–Cathodes put into electrolytic solution to get copper powder
 Pure, crystalline salts and their solutions in laboratory glassware. Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/stock-photo-pure-crystalline-salts-and-their-solutions-in-laboratory-glassware-92264190.html
Pure, crystalline salts and their solutions in laboratory glassware. Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/stock-photo-pure-crystalline-salts-and-their-solutions-in-laboratory-glassware-92264190.htmlRFFA2YWJ–Pure, crystalline salts and their solutions in laboratory glassware.
 Three colour salt solutions in laboratory glassware Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/stock-photo-three-colour-salt-solutions-in-laboratory-glassware-91504565.html
Three colour salt solutions in laboratory glassware Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/stock-photo-three-colour-salt-solutions-in-laboratory-glassware-91504565.htmlRFF8TB05–Three colour salt solutions in laboratory glassware
 . The Ontario high school physics. is placed at the bottom. The copper sulphate being denser than the zinc sulphate, sinks to the bottom, while the zinc sulphate floats above about the zinc plate. The copper sulphate solution is kept concentrated by placing crystals of the salt in a basket in the .outer vessel (Fig. 490), or at the bottom about the copper plate (Fig. 491). Tlie Daniell cell is capable of Fio. 491.—Gravity cell. Z, zinc plate; . ..A, zinc sulphate solution; B, copper giving a continuous current tor sulphate solution ; C, crystals of copper an indefinite period if the materials Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/the-ontario-high-school-physics-is-placed-at-the-bottom-the-copper-sulphate-being-denser-than-the-zinc-sulphate-sinks-to-the-bottom-while-the-zinc-sulphate-floats-above-about-the-zinc-plate-the-copper-sulphate-solution-is-kept-concentrated-by-placing-crystals-of-the-salt-in-a-basket-in-the-outer-vessel-fig-490-or-at-the-bottom-about-the-copper-plate-fig-491-tlie-daniell-cell-is-capable-of-fio-491gravity-cell-z-zinc-plate-a-zinc-sulphate-solution-b-copper-giving-a-continuous-current-tor-sulphate-solution-c-crystals-of-copper-an-indefinite-period-if-the-materials-image370376938.html
. The Ontario high school physics. is placed at the bottom. The copper sulphate being denser than the zinc sulphate, sinks to the bottom, while the zinc sulphate floats above about the zinc plate. The copper sulphate solution is kept concentrated by placing crystals of the salt in a basket in the .outer vessel (Fig. 490), or at the bottom about the copper plate (Fig. 491). Tlie Daniell cell is capable of Fio. 491.—Gravity cell. Z, zinc plate; . ..A, zinc sulphate solution; B, copper giving a continuous current tor sulphate solution ; C, crystals of copper an indefinite period if the materials Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/the-ontario-high-school-physics-is-placed-at-the-bottom-the-copper-sulphate-being-denser-than-the-zinc-sulphate-sinks-to-the-bottom-while-the-zinc-sulphate-floats-above-about-the-zinc-plate-the-copper-sulphate-solution-is-kept-concentrated-by-placing-crystals-of-the-salt-in-a-basket-in-the-outer-vessel-fig-490-or-at-the-bottom-about-the-copper-plate-fig-491-tlie-daniell-cell-is-capable-of-fio-491gravity-cell-z-zinc-plate-a-zinc-sulphate-solution-b-copper-giving-a-continuous-current-tor-sulphate-solution-c-crystals-of-copper-an-indefinite-period-if-the-materials-image370376938.htmlRM2CEG3FP–. The Ontario high school physics. is placed at the bottom. The copper sulphate being denser than the zinc sulphate, sinks to the bottom, while the zinc sulphate floats above about the zinc plate. The copper sulphate solution is kept concentrated by placing crystals of the salt in a basket in the .outer vessel (Fig. 490), or at the bottom about the copper plate (Fig. 491). Tlie Daniell cell is capable of Fio. 491.—Gravity cell. Z, zinc plate; . ..A, zinc sulphate solution; B, copper giving a continuous current tor sulphate solution ; C, crystals of copper an indefinite period if the materials
 Making copper sulphate crystals in a UK school science experiment Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/making-copper-sulphate-crystals-in-a-uk-school-science-experiment-image344146431.html
Making copper sulphate crystals in a UK school science experiment Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/making-copper-sulphate-crystals-in-a-uk-school-science-experiment-image344146431.htmlRF2AYW67Y–Making copper sulphate crystals in a UK school science experiment
 full frame background and texture of blue copper sulfate granules - close-up Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/full-frame-background-and-texture-of-blue-copper-sulfate-granules-close-up-image464743684.html
full frame background and texture of blue copper sulfate granules - close-up Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/full-frame-background-and-texture-of-blue-copper-sulfate-granules-close-up-image464743684.htmlRF2J02W8M–full frame background and texture of blue copper sulfate granules - close-up
 an iron nail being put into a beaker of copper sulphate solution in order to copper plate it iron displaces copper from solution Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/an-iron-nail-being-put-into-a-beaker-of-copper-sulphate-solution-in-image1418022.html
an iron nail being put into a beaker of copper sulphate solution in order to copper plate it iron displaces copper from solution Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/an-iron-nail-being-put-into-a-beaker-of-copper-sulphate-solution-in-image1418022.htmlRMANA327–an iron nail being put into a beaker of copper sulphate solution in order to copper plate it iron displaces copper from solution
 . The American fruit culturist, containing directions for the propagation and culture of all fruits adapted to the United States. Fruit-culture. 2l6 THE DISEASES OP FRUITS. tub or other wooden vessel filled with water. Hot water will greatly hasten the solution if it is desired. In preparing the full formula of sixty gallons, slowly pour a ten-gallon solution of the copper sulphate into twenty gallons of the lime wash, stirring thoroughly, after which the mixture is to be diluted to sixty gallons. For the application a force pump of some durable kind at- tached to a tank and mounted upon wheel Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/the-american-fruit-culturist-containing-directions-for-the-propagation-and-culture-of-all-fruits-adapted-to-the-united-states-fruit-culture-2l6-the-diseases-op-fruits-tub-or-other-wooden-vessel-filled-with-water-hot-water-will-greatly-hasten-the-solution-if-it-is-desired-in-preparing-the-full-formula-of-sixty-gallons-slowly-pour-a-ten-gallon-solution-of-the-copper-sulphate-into-twenty-gallons-of-the-lime-wash-stirring-thoroughly-after-which-the-mixture-is-to-be-diluted-to-sixty-gallons-for-the-application-a-force-pump-of-some-durable-kind-at-tached-to-a-tank-and-mounted-upon-wheel-image216359635.html
. The American fruit culturist, containing directions for the propagation and culture of all fruits adapted to the United States. Fruit-culture. 2l6 THE DISEASES OP FRUITS. tub or other wooden vessel filled with water. Hot water will greatly hasten the solution if it is desired. In preparing the full formula of sixty gallons, slowly pour a ten-gallon solution of the copper sulphate into twenty gallons of the lime wash, stirring thoroughly, after which the mixture is to be diluted to sixty gallons. For the application a force pump of some durable kind at- tached to a tank and mounted upon wheel Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/the-american-fruit-culturist-containing-directions-for-the-propagation-and-culture-of-all-fruits-adapted-to-the-united-states-fruit-culture-2l6-the-diseases-op-fruits-tub-or-other-wooden-vessel-filled-with-water-hot-water-will-greatly-hasten-the-solution-if-it-is-desired-in-preparing-the-full-formula-of-sixty-gallons-slowly-pour-a-ten-gallon-solution-of-the-copper-sulphate-into-twenty-gallons-of-the-lime-wash-stirring-thoroughly-after-which-the-mixture-is-to-be-diluted-to-sixty-gallons-for-the-application-a-force-pump-of-some-durable-kind-at-tached-to-a-tank-and-mounted-upon-wheel-image216359635.htmlRMPG00WR–. The American fruit culturist, containing directions for the propagation and culture of all fruits adapted to the United States. Fruit-culture. 2l6 THE DISEASES OP FRUITS. tub or other wooden vessel filled with water. Hot water will greatly hasten the solution if it is desired. In preparing the full formula of sixty gallons, slowly pour a ten-gallon solution of the copper sulphate into twenty gallons of the lime wash, stirring thoroughly, after which the mixture is to be diluted to sixty gallons. For the application a force pump of some durable kind at- tached to a tank and mounted upon wheel
 Petri dishes of copper sulphate solution isolated on white background Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/stock-image-petri-dishes-of-copper-sulphate-solution-isolated-on-white-background-162029316.html
Petri dishes of copper sulphate solution isolated on white background Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/stock-image-petri-dishes-of-copper-sulphate-solution-isolated-on-white-background-162029316.htmlRFKBH218–Petri dishes of copper sulphate solution isolated on white background
 Glass ampoules containing iron supplement solution to treat anaemia. Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/glass-ampoules-containing-iron-supplement-solution-to-treat-anaemia-image470064377.html
Glass ampoules containing iron supplement solution to treat anaemia. Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/glass-ampoules-containing-iron-supplement-solution-to-treat-anaemia-image470064377.htmlRF2J8N7WD–Glass ampoules containing iron supplement solution to treat anaemia.
