Get 20% off Images & Videos—Ends Sunday! Use code: FLASH20%
Quick filters:
Iron filings and sulphur Stock Photos and Images
 The chemical mixture of iron filings and sulphur in an ignition tube, being separated with a magnet, prior to being heated to create iron sulphide Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/the-chemical-mixture-of-iron-filings-and-sulphur-in-an-ignition-tube-being-separated-with-a-magnet-prior-to-being-heated-to-create-iron-sulphide-image240978082.html
The chemical mixture of iron filings and sulphur in an ignition tube, being separated with a magnet, prior to being heated to create iron sulphide Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/the-chemical-mixture-of-iron-filings-and-sulphur-in-an-ignition-tube-being-separated-with-a-magnet-prior-to-being-heated-to-create-iron-sulphide-image240978082.htmlRFT01E02–The chemical mixture of iron filings and sulphur in an ignition tube, being separated with a magnet, prior to being heated to create iron sulphide
 Separation of sulphur and iron filings using a magnet Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/stock-photo-separation-of-sulphur-and-iron-filings-using-a-magnet-94490400.html
Separation of sulphur and iron filings using a magnet Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/stock-photo-separation-of-sulphur-and-iron-filings-using-a-magnet-94490400.htmlRMFDMBD4–Separation of sulphur and iron filings using a magnet
 Iron sulphate in an ignition tube produced by heating sulphur with iron filings Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/iron-sulphate-in-an-ignition-tube-produced-by-heating-sulphur-with-iron-filings-image336542415.html
Iron sulphate in an ignition tube produced by heating sulphur with iron filings Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/iron-sulphate-in-an-ignition-tube-produced-by-heating-sulphur-with-iron-filings-image336542415.htmlRF2AFER7Y–Iron sulphate in an ignition tube produced by heating sulphur with iron filings
 Iron filings and sulphur labelled Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/stock-photo-iron-filings-and-sulphur-labelled-16490298.html
Iron filings and sulphur labelled Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/stock-photo-iron-filings-and-sulphur-labelled-16490298.htmlRMAW7CXK–Iron filings and sulphur labelled
 Iron filings and sulphur powder on glass dish Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/iron-filings-and-sulphur-powder-on-glass-dish-image216032491.html
Iron filings and sulphur powder on glass dish Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/iron-filings-and-sulphur-powder-on-glass-dish-image216032491.htmlRMPFD3J3–Iron filings and sulphur powder on glass dish
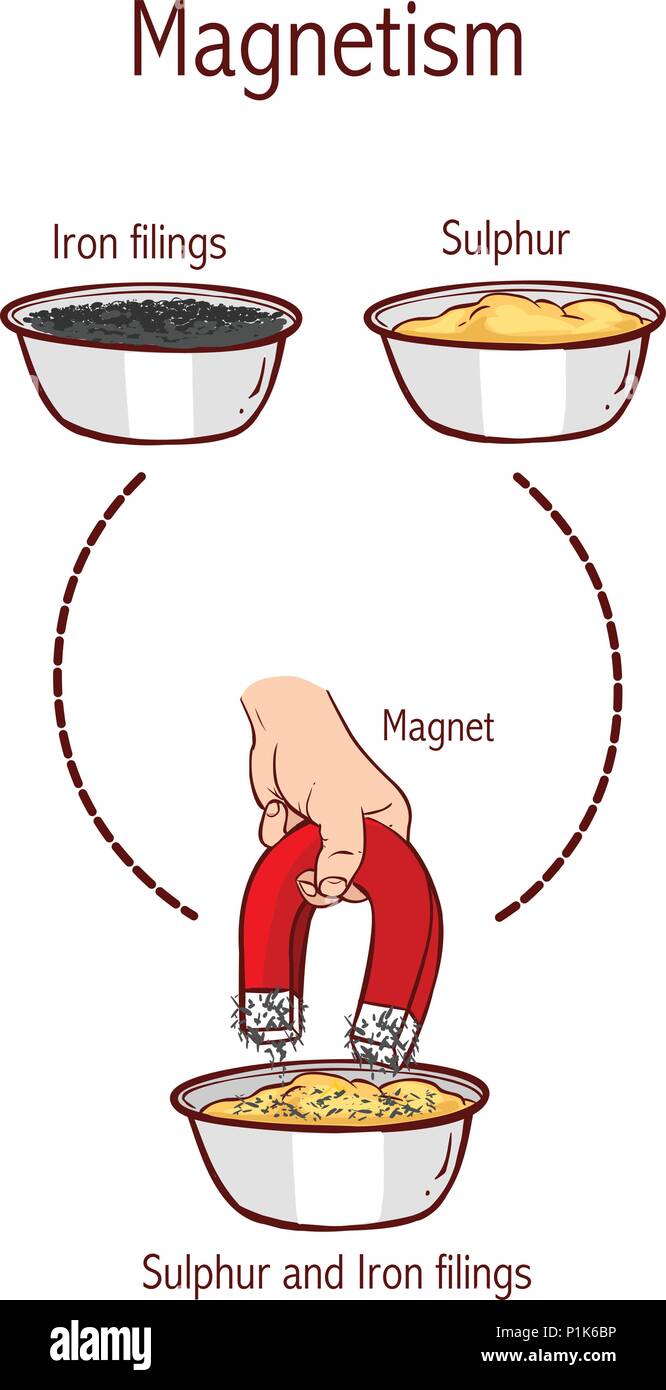 Separation of sulphur and iron filings using a magnet Stock Vectorhttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/separation-of-sulphur-and-iron-filings-using-a-magnet-image207561194.html
Separation of sulphur and iron filings using a magnet Stock Vectorhttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/separation-of-sulphur-and-iron-filings-using-a-magnet-image207561194.htmlRFP1K6BP–Separation of sulphur and iron filings using a magnet
 Elements of chemistry : including the applications of the science in the arts . mixture of 4 parts of coarse sulphur and 7 of iron J filings or borings in a covered stoneware or cast-iron crucible. The sulphide of iron, thus obtained, isbroken into lumps, and acted upon by diluted sul-phuric acid in a gas-bottle (fig. 141), exactly as zincis treated in the preparation of hydrogen gas.Hydrosulphuric acid is evolved without the appli-cation of heat, and should be collected over waterat 80° or 90°; or if collected in a gasometer orgasholder, the latter may be filled with brine, inwhich this gas Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/elements-of-chemistry-including-the-applications-of-the-science-in-the-arts-mixture-of-4-parts-of-coarse-sulphur-and-7-of-iron-j-filings-or-borings-in-a-covered-stoneware-or-cast-iron-crucible-the-sulphide-of-iron-thus-obtained-isbroken-into-lumps-and-acted-upon-by-diluted-sul-phuric-acid-in-a-gas-bottle-fig-141-exactly-as-zincis-treated-in-the-preparation-of-hydrogen-gashydrosulphuric-acid-is-evolved-without-the-appli-cation-of-heat-and-should-be-collected-over-waterat-80-or-90-or-if-collected-in-a-gasometer-orgasholder-the-latter-may-be-filled-with-brine-inwhich-this-gas-image339439151.html
Elements of chemistry : including the applications of the science in the arts . mixture of 4 parts of coarse sulphur and 7 of iron J filings or borings in a covered stoneware or cast-iron crucible. The sulphide of iron, thus obtained, isbroken into lumps, and acted upon by diluted sul-phuric acid in a gas-bottle (fig. 141), exactly as zincis treated in the preparation of hydrogen gas.Hydrosulphuric acid is evolved without the appli-cation of heat, and should be collected over waterat 80° or 90°; or if collected in a gasometer orgasholder, the latter may be filled with brine, inwhich this gas Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/elements-of-chemistry-including-the-applications-of-the-science-in-the-arts-mixture-of-4-parts-of-coarse-sulphur-and-7-of-iron-j-filings-or-borings-in-a-covered-stoneware-or-cast-iron-crucible-the-sulphide-of-iron-thus-obtained-isbroken-into-lumps-and-acted-upon-by-diluted-sul-phuric-acid-in-a-gas-bottle-fig-141-exactly-as-zincis-treated-in-the-preparation-of-hydrogen-gashydrosulphuric-acid-is-evolved-without-the-appli-cation-of-heat-and-should-be-collected-over-waterat-80-or-90-or-if-collected-in-a-gasometer-orgasholder-the-latter-may-be-filled-with-brine-inwhich-this-gas-image339439151.htmlRM2AM6P2R–Elements of chemistry : including the applications of the science in the arts . mixture of 4 parts of coarse sulphur and 7 of iron J filings or borings in a covered stoneware or cast-iron crucible. The sulphide of iron, thus obtained, isbroken into lumps, and acted upon by diluted sul-phuric acid in a gas-bottle (fig. 141), exactly as zincis treated in the preparation of hydrogen gas.Hydrosulphuric acid is evolved without the appli-cation of heat, and should be collected over waterat 80° or 90°; or if collected in a gasometer orgasholder, the latter may be filled with brine, inwhich this gas
 weight of tube with iron filings and sulphur AFTER heating and reacting see AWT79P for BEFORE heating Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/weight-of-tube-with-iron-filings-and-sulphur-after-heating-and-reacting-image1417917.html
weight of tube with iron filings and sulphur AFTER heating and reacting see AWT79P for BEFORE heating Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/weight-of-tube-with-iron-filings-and-sulphur-after-heating-and-reacting-image1417917.htmlRMANA2BE–weight of tube with iron filings and sulphur AFTER heating and reacting see AWT79P for BEFORE heating
 Removing iron filings from sulphur mixture with magnet, close-up Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/removing-iron-filings-from-sulphur-mixture-with-magnet-close-up-image216032508.html
Removing iron filings from sulphur mixture with magnet, close-up Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/removing-iron-filings-from-sulphur-mixture-with-magnet-close-up-image216032508.htmlRMPFD3JM–Removing iron filings from sulphur mixture with magnet, close-up
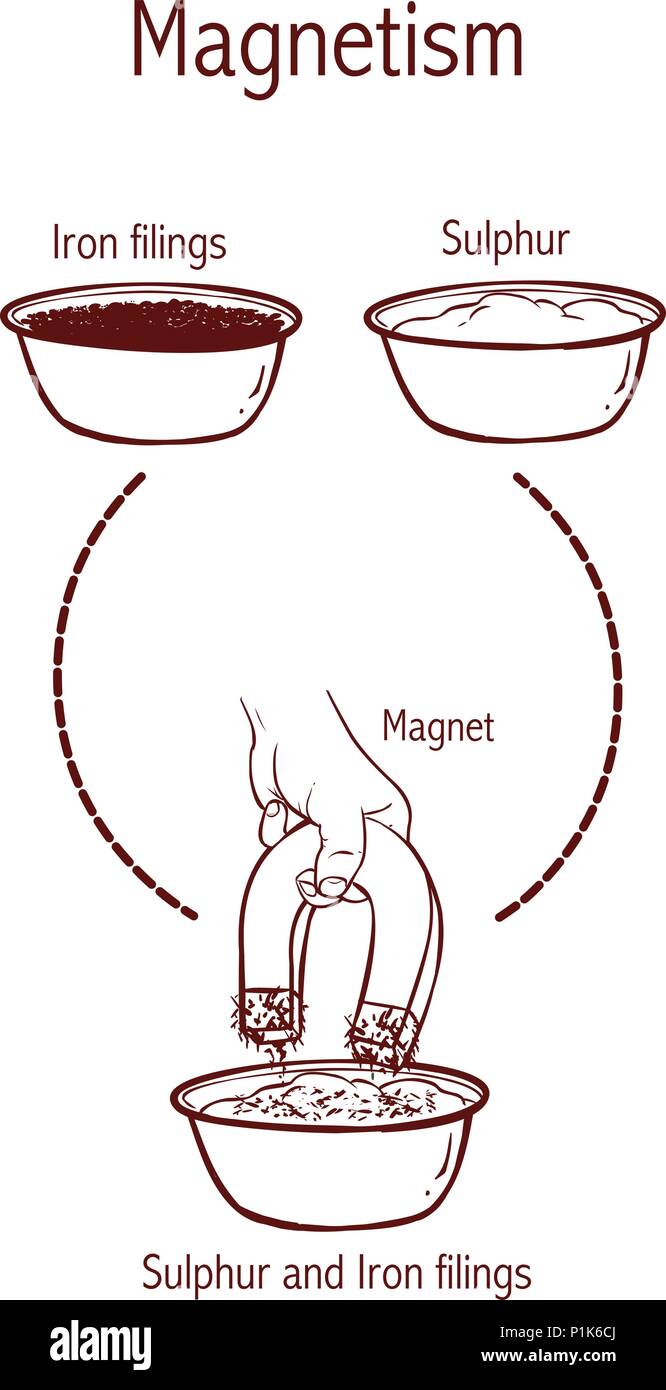 Separation of sulphur and iron filings using a magnet Stock Vectorhttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/separation-of-sulphur-and-iron-filings-using-a-magnet-image207561218.html
Separation of sulphur and iron filings using a magnet Stock Vectorhttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/separation-of-sulphur-and-iron-filings-using-a-magnet-image207561218.htmlRFP1K6CJ–Separation of sulphur and iron filings using a magnet
 Appleton's dictionary of machines, mechanics, engine-work, and engineering . ng of the joints is lead; with hot air, an iron cement mustbo used. This cement is made of 99 parts of iron filings, sifted fine, and 1 part of powdered sal am-noniac, intimately mixed, dry. When used, as much water is added as will make a stiff paste. Flow-ers of sulphur, sometimes recommended, in no way contributes to the efficiency of the mixture, butrather to the contrary. The pipes are sometimes laid under ground and covered over; but this mode is not to he recom-mended ; they should be always accessible. If hot Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/appletons-dictionary-of-machines-mechanics-engine-work-and-engineering-ng-of-the-joints-is-lead-with-hot-air-an-iron-cement-mustbo-used-this-cement-is-made-of-99-parts-of-iron-filings-sifted-fine-and-1-part-of-powdered-sal-am-noniac-intimately-mixed-dry-when-used-as-much-water-is-added-as-will-make-a-stiff-paste-flow-ers-of-sulphur-sometimes-recommended-in-no-way-contributes-to-the-efficiency-of-the-mixture-butrather-to-the-contrary-the-pipes-are-sometimes-laid-under-ground-and-covered-over-but-this-mode-is-not-to-he-recom-mended-they-should-be-always-accessible-if-hot-image338103842.html
Appleton's dictionary of machines, mechanics, engine-work, and engineering . ng of the joints is lead; with hot air, an iron cement mustbo used. This cement is made of 99 parts of iron filings, sifted fine, and 1 part of powdered sal am-noniac, intimately mixed, dry. When used, as much water is added as will make a stiff paste. Flow-ers of sulphur, sometimes recommended, in no way contributes to the efficiency of the mixture, butrather to the contrary. The pipes are sometimes laid under ground and covered over; but this mode is not to he recom-mended ; they should be always accessible. If hot Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/appletons-dictionary-of-machines-mechanics-engine-work-and-engineering-ng-of-the-joints-is-lead-with-hot-air-an-iron-cement-mustbo-used-this-cement-is-made-of-99-parts-of-iron-filings-sifted-fine-and-1-part-of-powdered-sal-am-noniac-intimately-mixed-dry-when-used-as-much-water-is-added-as-will-make-a-stiff-paste-flow-ers-of-sulphur-sometimes-recommended-in-no-way-contributes-to-the-efficiency-of-the-mixture-butrather-to-the-contrary-the-pipes-are-sometimes-laid-under-ground-and-covered-over-but-this-mode-is-not-to-he-recom-mended-they-should-be-always-accessible-if-hot-image338103842.htmlRM2AJ1XW6–Appleton's dictionary of machines, mechanics, engine-work, and engineering . ng of the joints is lead; with hot air, an iron cement mustbo used. This cement is made of 99 parts of iron filings, sifted fine, and 1 part of powdered sal am-noniac, intimately mixed, dry. When used, as much water is added as will make a stiff paste. Flow-ers of sulphur, sometimes recommended, in no way contributes to the efficiency of the mixture, butrather to the contrary. The pipes are sometimes laid under ground and covered over; but this mode is not to he recom-mended ; they should be always accessible. If hot
 Weighing mixture of sulphur and iron filings on a top pan balance BEFORE heating see ANA2BE for AFTER heating Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/stock-photo-weighing-mixture-of-sulphur-and-iron-filings-on-a-top-pan-balance-16648353.html
Weighing mixture of sulphur and iron filings on a top pan balance BEFORE heating see ANA2BE for AFTER heating Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/stock-photo-weighing-mixture-of-sulphur-and-iron-filings-on-a-top-pan-balance-16648353.htmlRMAWT79P–Weighing mixture of sulphur and iron filings on a top pan balance BEFORE heating see ANA2BE for AFTER heating
 . Essentials of medical and clinical chemistry. With laboratory exercises . —Fe2(S04)3.— Tersiclphate is made by adding iron and gunpowder into alcohol burning m a dinner-plate and note that theiron burns with bright scintillations, while the gunpowder falls through theflame and is not ignited uniil the alcohol is burned away to the surface of theplate. (***) Make an iron gunpowder by mixing I Gm. of reduced iron, 2 Grn.of sulphur and 3 Gm. of KN03, and note that it burns as quickly and morebrilliantly than ordinary gunpowder. 2 5Ferrous Salts. Dissolve iron filings in warm dilute H2SO,. Allow Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/essentials-of-medical-and-clinical-chemistry-with-laboratory-exercises-fe2s043-tersiclphate-is-made-by-adding-iron-and-gunpowder-into-alcohol-burning-m-a-dinner-plate-and-note-that-theiron-burns-with-bright-scintillations-while-the-gunpowder-falls-through-theflame-and-is-not-ignited-uniil-the-alcohol-is-burned-away-to-the-surface-of-theplate-make-an-iron-gunpowder-by-mixing-i-gm-of-reduced-iron-2-grnof-sulphur-and-3-gm-of-kn03-and-note-that-it-burns-as-quickly-and-morebrilliantly-than-ordinary-gunpowder-2-5ferrous-salts-dissolve-iron-filings-in-warm-dilute-h2so-allow-image372454515.html
. Essentials of medical and clinical chemistry. With laboratory exercises . —Fe2(S04)3.— Tersiclphate is made by adding iron and gunpowder into alcohol burning m a dinner-plate and note that theiron burns with bright scintillations, while the gunpowder falls through theflame and is not ignited uniil the alcohol is burned away to the surface of theplate. (***) Make an iron gunpowder by mixing I Gm. of reduced iron, 2 Grn.of sulphur and 3 Gm. of KN03, and note that it burns as quickly and morebrilliantly than ordinary gunpowder. 2 5Ferrous Salts. Dissolve iron filings in warm dilute H2SO,. Allow Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/essentials-of-medical-and-clinical-chemistry-with-laboratory-exercises-fe2s043-tersiclphate-is-made-by-adding-iron-and-gunpowder-into-alcohol-burning-m-a-dinner-plate-and-note-that-theiron-burns-with-bright-scintillations-while-the-gunpowder-falls-through-theflame-and-is-not-ignited-uniil-the-alcohol-is-burned-away-to-the-surface-of-theplate-make-an-iron-gunpowder-by-mixing-i-gm-of-reduced-iron-2-grnof-sulphur-and-3-gm-of-kn03-and-note-that-it-burns-as-quickly-and-morebrilliantly-than-ordinary-gunpowder-2-5ferrous-salts-dissolve-iron-filings-in-warm-dilute-h2so-allow-image372454515.htmlRM2CHXNEY–. Essentials of medical and clinical chemistry. With laboratory exercises . —Fe2(S04)3.— Tersiclphate is made by adding iron and gunpowder into alcohol burning m a dinner-plate and note that theiron burns with bright scintillations, while the gunpowder falls through theflame and is not ignited uniil the alcohol is burned away to the surface of theplate. (***) Make an iron gunpowder by mixing I Gm. of reduced iron, 2 Grn.of sulphur and 3 Gm. of KN03, and note that it burns as quickly and morebrilliantly than ordinary gunpowder. 2 5Ferrous Salts. Dissolve iron filings in warm dilute H2SO,. Allow
 . Scientific American Volume 06 Number 21 (May 1862) . ld render it a nuisance toexhaust the tank in the public streets of a city. Cement ion .Joints of Petboleum Stills.—j-t corre-spondent states that refiners of petroleum are muchtroubled to obtain a suitable cement for their stillsso as to form ;i tight and durable joint. The cementused for cast iron, made with iron filings, sal ammo-niac and sulphur, has been tried and found wanting ;Lead makes a tight joint, but is liable to melt outwith the high heat used. Copper also makes a tightjoint, but it soon corrodes and becomes useless. Va-rious Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/scientific-american-volume-06-number-21-may-1862-ld-render-it-a-nuisance-toexhaust-the-tank-in-the-public-streets-of-a-city-cement-ion-joints-of-petboleum-stillsj-t-corre-spondent-states-that-refiners-of-petroleum-are-muchtroubled-to-obtain-a-suitable-cement-for-their-stillsso-as-to-form-i-tight-and-durable-joint-the-cementused-for-cast-iron-made-with-iron-filings-sal-ammo-niac-and-sulphur-has-been-tried-and-found-wanting-lead-makes-a-tight-joint-but-is-liable-to-melt-outwith-the-high-heat-used-copper-also-makes-a-tightjoint-but-it-soon-corrodes-and-becomes-useless-va-rious-image372235767.html
. Scientific American Volume 06 Number 21 (May 1862) . ld render it a nuisance toexhaust the tank in the public streets of a city. Cement ion .Joints of Petboleum Stills.—j-t corre-spondent states that refiners of petroleum are muchtroubled to obtain a suitable cement for their stillsso as to form ;i tight and durable joint. The cementused for cast iron, made with iron filings, sal ammo-niac and sulphur, has been tried and found wanting ;Lead makes a tight joint, but is liable to melt outwith the high heat used. Copper also makes a tightjoint, but it soon corrodes and becomes useless. Va-rious Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/scientific-american-volume-06-number-21-may-1862-ld-render-it-a-nuisance-toexhaust-the-tank-in-the-public-streets-of-a-city-cement-ion-joints-of-petboleum-stillsj-t-corre-spondent-states-that-refiners-of-petroleum-are-muchtroubled-to-obtain-a-suitable-cement-for-their-stillsso-as-to-form-i-tight-and-durable-joint-the-cementused-for-cast-iron-made-with-iron-filings-sal-ammo-niac-and-sulphur-has-been-tried-and-found-wanting-lead-makes-a-tight-joint-but-is-liable-to-melt-outwith-the-high-heat-used-copper-also-makes-a-tightjoint-but-it-soon-corrodes-and-becomes-useless-va-rious-image372235767.htmlRM2CHGPEF–. Scientific American Volume 06 Number 21 (May 1862) . ld render it a nuisance toexhaust the tank in the public streets of a city. Cement ion .Joints of Petboleum Stills.—j-t corre-spondent states that refiners of petroleum are muchtroubled to obtain a suitable cement for their stillsso as to form ;i tight and durable joint. The cementused for cast iron, made with iron filings, sal ammo-niac and sulphur, has been tried and found wanting ;Lead makes a tight joint, but is liable to melt outwith the high heat used. Copper also makes a tightjoint, but it soon corrodes and becomes useless. Va-rious
 . Essentials of medical and clinical chemistry. With laboratory exercises . lphur). 57 In a small glass flask, a little sulphur is heated to boiling. If now a bun-dle of fine copper wire or a piece of sodium, in a combustion spoon, be previ-ously heated and then lowered into the vapor, it burns brilliantly. 58 Mix in a dish equal parts of iron filings and flowers of sulphur.: moistenwith water and set aside. Within a half hour it gets hot, vaporizes the water,and is converted into a black mass of FeS. 59 Into a side-neck test-tube, or better a flask with funnel and delivery tube,Fig. 19, put a Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/essentials-of-medical-and-clinical-chemistry-with-laboratory-exercises-lphur-57-in-a-small-glass-flask-a-little-sulphur-is-heated-to-boiling-if-now-a-bun-dle-of-fine-copper-wire-or-a-piece-of-sodium-in-a-combustion-spoon-be-previ-ously-heated-and-then-lowered-into-the-vapor-it-burns-brilliantly-58-mix-in-a-dish-equal-parts-of-iron-filings-and-flowers-of-sulphur-moistenwith-water-and-set-aside-within-a-half-hour-it-gets-hot-vaporizes-the-waterand-is-converted-into-a-black-mass-of-fes-59-into-a-side-neck-test-tube-or-better-a-flask-with-funnel-and-delivery-tubefig-19-put-a-image372472214.html
. Essentials of medical and clinical chemistry. With laboratory exercises . lphur). 57 In a small glass flask, a little sulphur is heated to boiling. If now a bun-dle of fine copper wire or a piece of sodium, in a combustion spoon, be previ-ously heated and then lowered into the vapor, it burns brilliantly. 58 Mix in a dish equal parts of iron filings and flowers of sulphur.: moistenwith water and set aside. Within a half hour it gets hot, vaporizes the water,and is converted into a black mass of FeS. 59 Into a side-neck test-tube, or better a flask with funnel and delivery tube,Fig. 19, put a Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/essentials-of-medical-and-clinical-chemistry-with-laboratory-exercises-lphur-57-in-a-small-glass-flask-a-little-sulphur-is-heated-to-boiling-if-now-a-bun-dle-of-fine-copper-wire-or-a-piece-of-sodium-in-a-combustion-spoon-be-previ-ously-heated-and-then-lowered-into-the-vapor-it-burns-brilliantly-58-mix-in-a-dish-equal-parts-of-iron-filings-and-flowers-of-sulphur-moistenwith-water-and-set-aside-within-a-half-hour-it-gets-hot-vaporizes-the-waterand-is-converted-into-a-black-mass-of-fes-59-into-a-side-neck-test-tube-or-better-a-flask-with-funnel-and-delivery-tubefig-19-put-a-image372472214.htmlRM2CHYG32–. Essentials of medical and clinical chemistry. With laboratory exercises . lphur). 57 In a small glass flask, a little sulphur is heated to boiling. If now a bun-dle of fine copper wire or a piece of sodium, in a combustion spoon, be previ-ously heated and then lowered into the vapor, it burns brilliantly. 58 Mix in a dish equal parts of iron filings and flowers of sulphur.: moistenwith water and set aside. Within a half hour it gets hot, vaporizes the water,and is converted into a black mass of FeS. 59 Into a side-neck test-tube, or better a flask with funnel and delivery tube,Fig. 19, put a