Quick filters:
Leaf micrograph section Stock Photos and Images
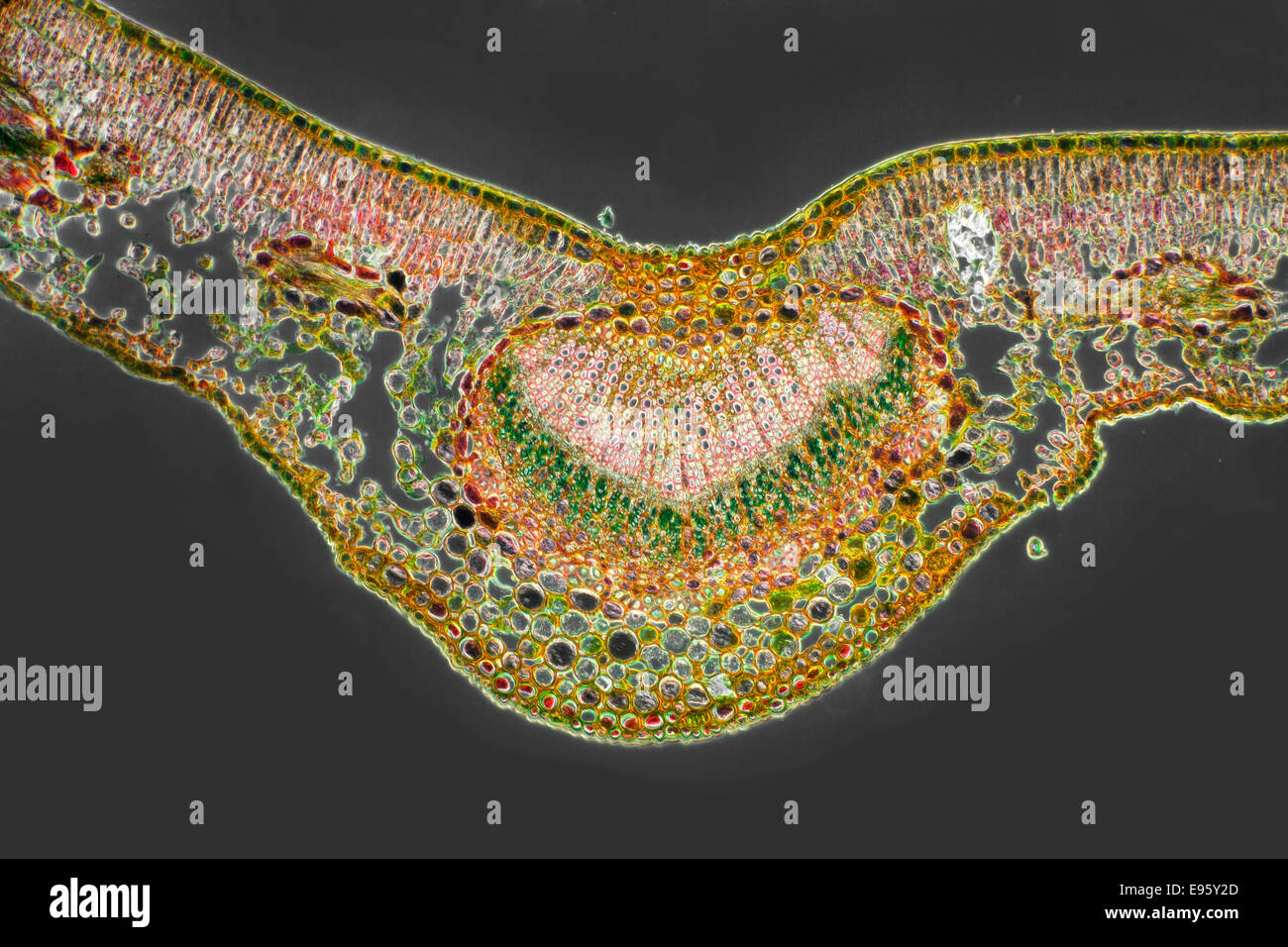 Berberis vulgaris, Barberry leaf TS stained section central vein, darkfield photomicrograph Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/stock-photo-berberis-vulgaris-barberry-leaf-ts-stained-section-central-vein-darkfield-74504373.html
Berberis vulgaris, Barberry leaf TS stained section central vein, darkfield photomicrograph Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/stock-photo-berberis-vulgaris-barberry-leaf-ts-stained-section-central-vein-darkfield-74504373.htmlRME95Y2D–Berberis vulgaris, Barberry leaf TS stained section central vein, darkfield photomicrograph
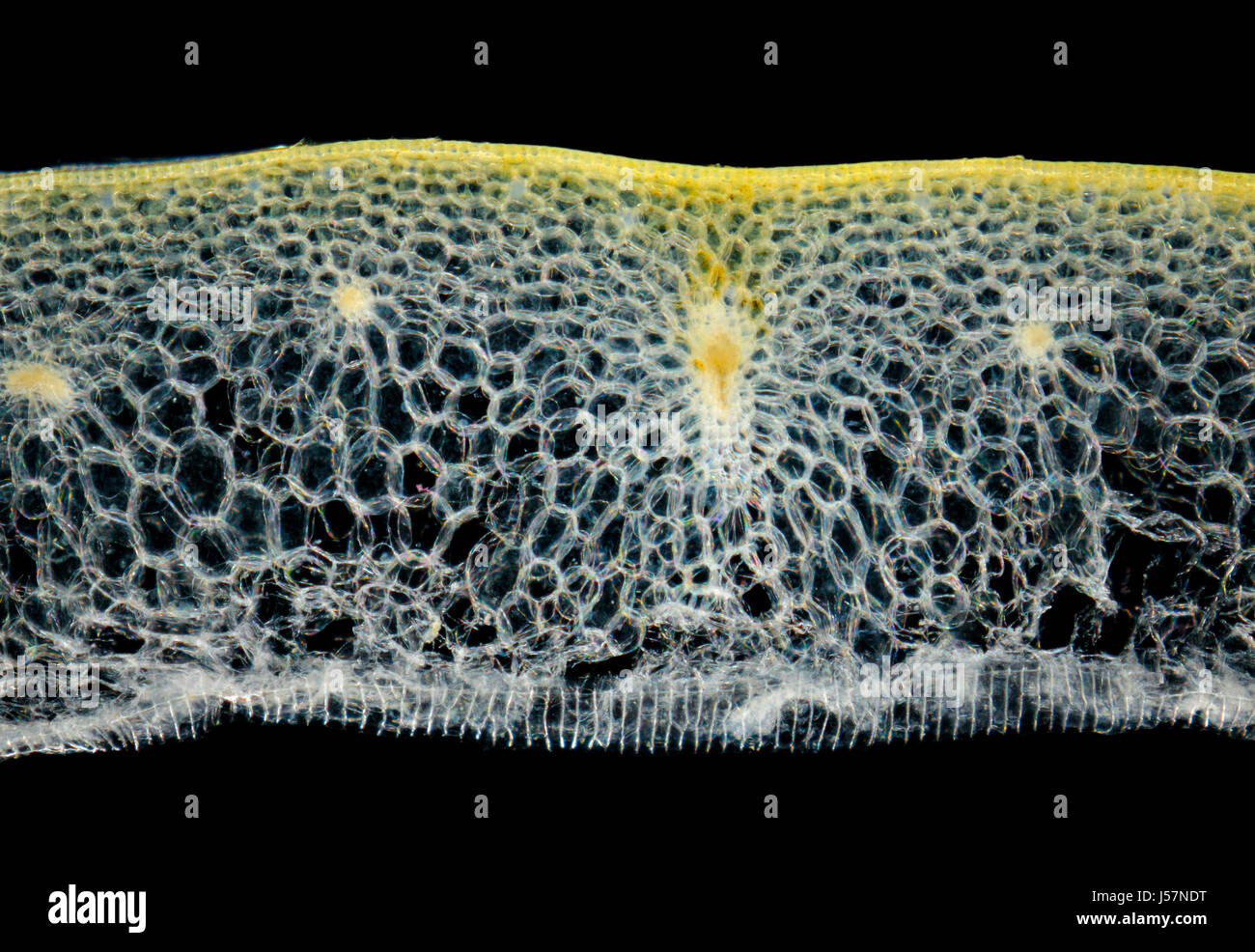 Microscopic view of a Onion (Allium cepa) bulb leaf cross-section. Darkfield illumination. Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/stock-photo-microscopic-view-of-a-onion-allium-cepa-bulb-leaf-cross-section-darkfield-140926740.html
Microscopic view of a Onion (Allium cepa) bulb leaf cross-section. Darkfield illumination. Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/stock-photo-microscopic-view-of-a-onion-allium-cepa-bulb-leaf-cross-section-darkfield-140926740.htmlRFJ57NDT–Microscopic view of a Onion (Allium cepa) bulb leaf cross-section. Darkfield illumination.
 Light Micrograph (LM) of a transverse section of a leaf of a Tulip (Tulipa sp.) showing stomata, magnification x1200 Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/stock-photo-light-micrograph-lm-of-a-transverse-section-of-a-leaf-of-a-tulip-tulipa-31611971.html
Light Micrograph (LM) of a transverse section of a leaf of a Tulip (Tulipa sp.) showing stomata, magnification x1200 Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/stock-photo-light-micrograph-lm-of-a-transverse-section-of-a-leaf-of-a-tulip-tulipa-31611971.htmlRMBRC1AY–Light Micrograph (LM) of a transverse section of a leaf of a Tulip (Tulipa sp.) showing stomata, magnification x1200
 Pine leaf, cross section, 20X light micrograph. Pine tree needle under the microscope. Epidermis cells, cuticle, mesophyll, resin canals, etc. Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/pine-leaf-cross-section-20x-light-micrograph-pine-tree-needle-under-the-microscope-epidermis-cells-cuticle-mesophyll-resin-canals-etc-image570394016.html
Pine leaf, cross section, 20X light micrograph. Pine tree needle under the microscope. Epidermis cells, cuticle, mesophyll, resin canals, etc. Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/pine-leaf-cross-section-20x-light-micrograph-pine-tree-needle-under-the-microscope-epidermis-cells-cuticle-mesophyll-resin-canals-etc-image570394016.htmlRF2T3YKAT–Pine leaf, cross section, 20X light micrograph. Pine tree needle under the microscope. Epidermis cells, cuticle, mesophyll, resin canals, etc.
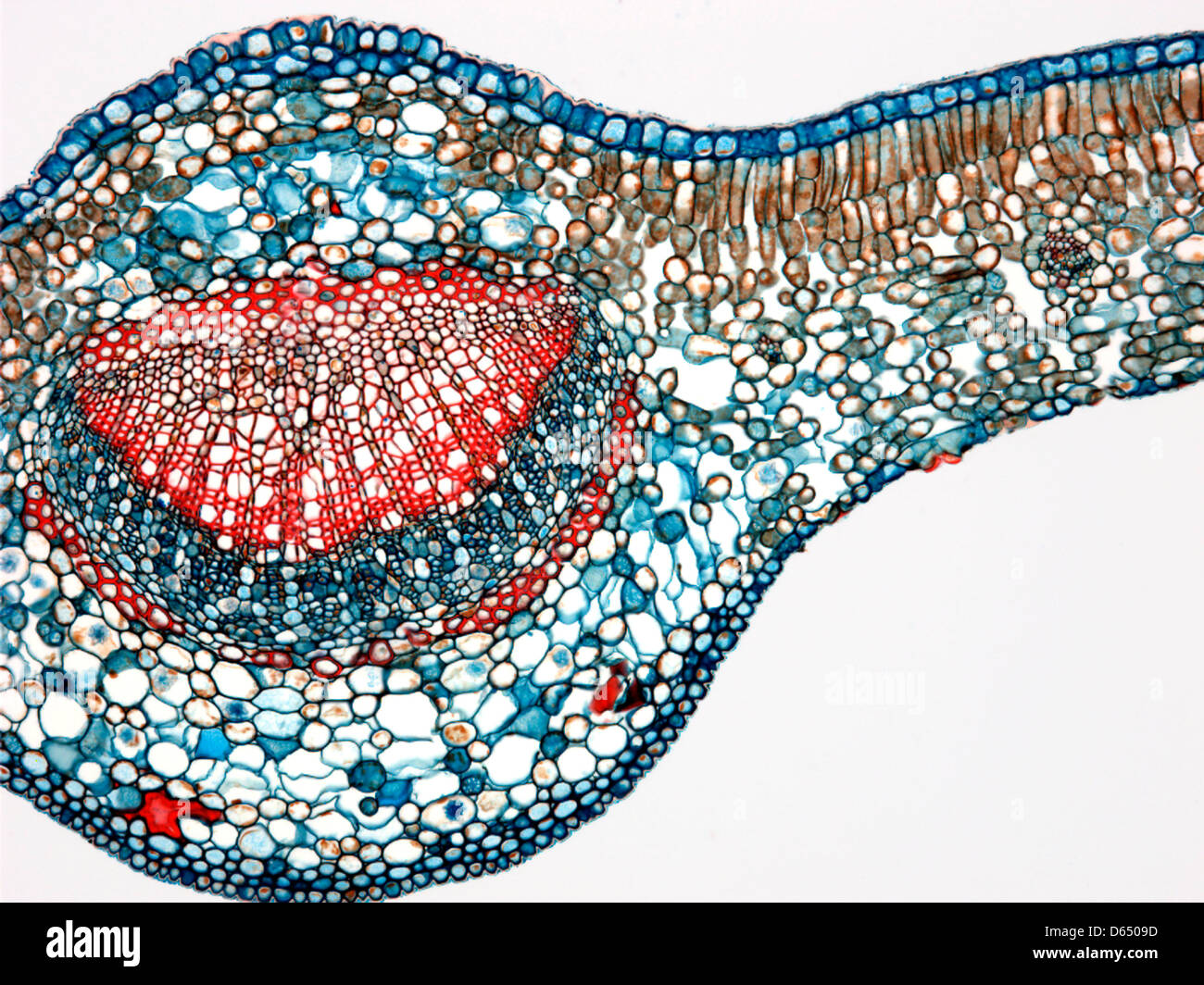 Tea leaf, light micrograph Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/stock-photo-tea-leaf-light-micrograph-55429065.html
Tea leaf, light micrograph Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/stock-photo-tea-leaf-light-micrograph-55429065.htmlRFD6509D–Tea leaf, light micrograph
 Pine tree leaf, cross section, 20X light micrograph. Needle of Pinus, under microscope. Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/pine-tree-leaf-cross-section-20x-light-micrograph-needle-of-pinus-under-microscope-image545723854.html
Pine tree leaf, cross section, 20X light micrograph. Needle of Pinus, under microscope. Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/pine-tree-leaf-cross-section-20x-light-micrograph-needle-of-pinus-under-microscope-image545723854.htmlRF2PKRT9J–Pine tree leaf, cross section, 20X light micrograph. Needle of Pinus, under microscope.
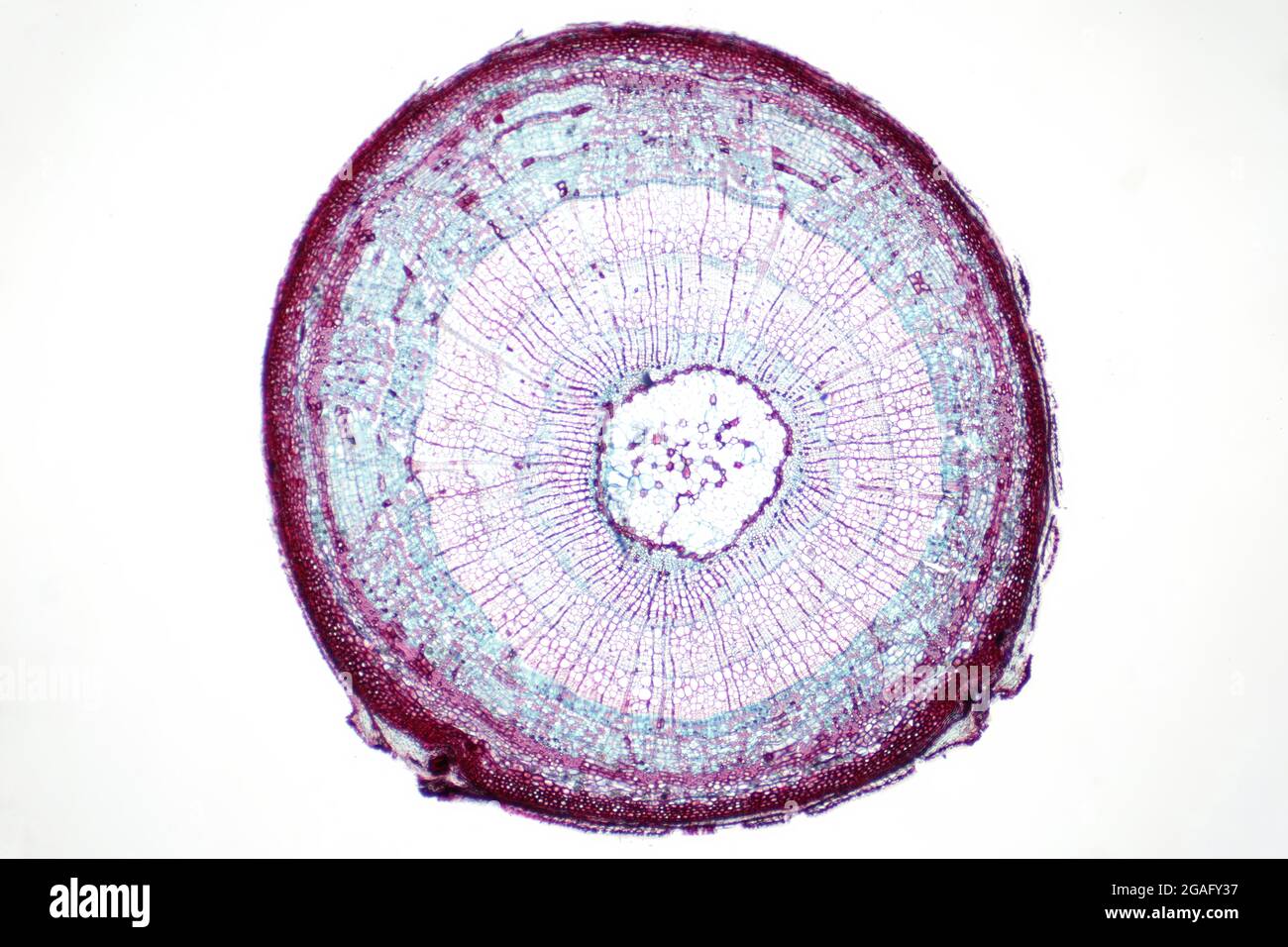
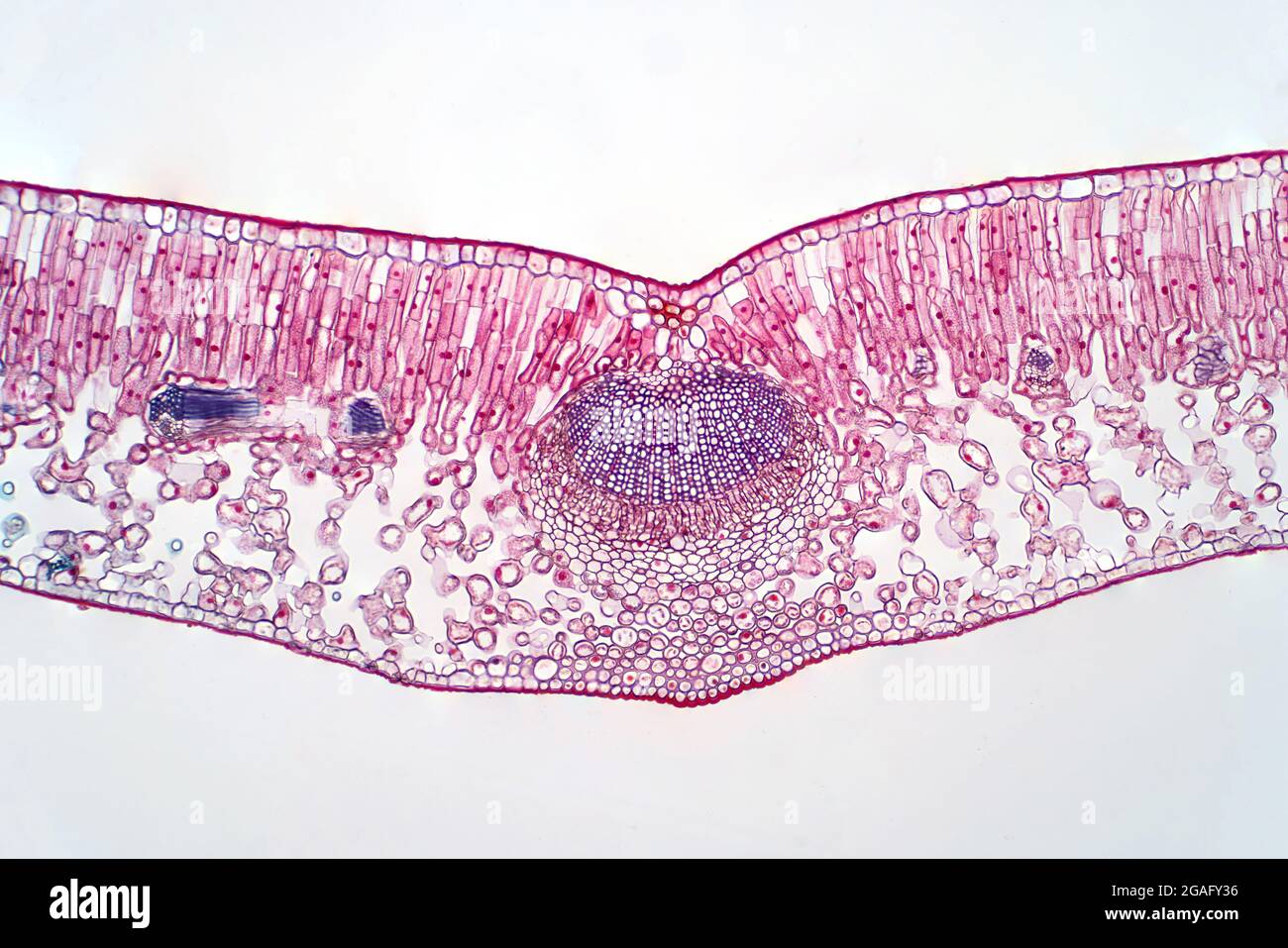 Cross section of a leaf, light micrograph Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/cross-section-of-a-leaf-light-micrograph-image436756298.html
Cross section of a leaf, light micrograph Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/cross-section-of-a-leaf-light-micrograph-image436756298.htmlRF2GAFY36–Cross section of a leaf, light micrograph
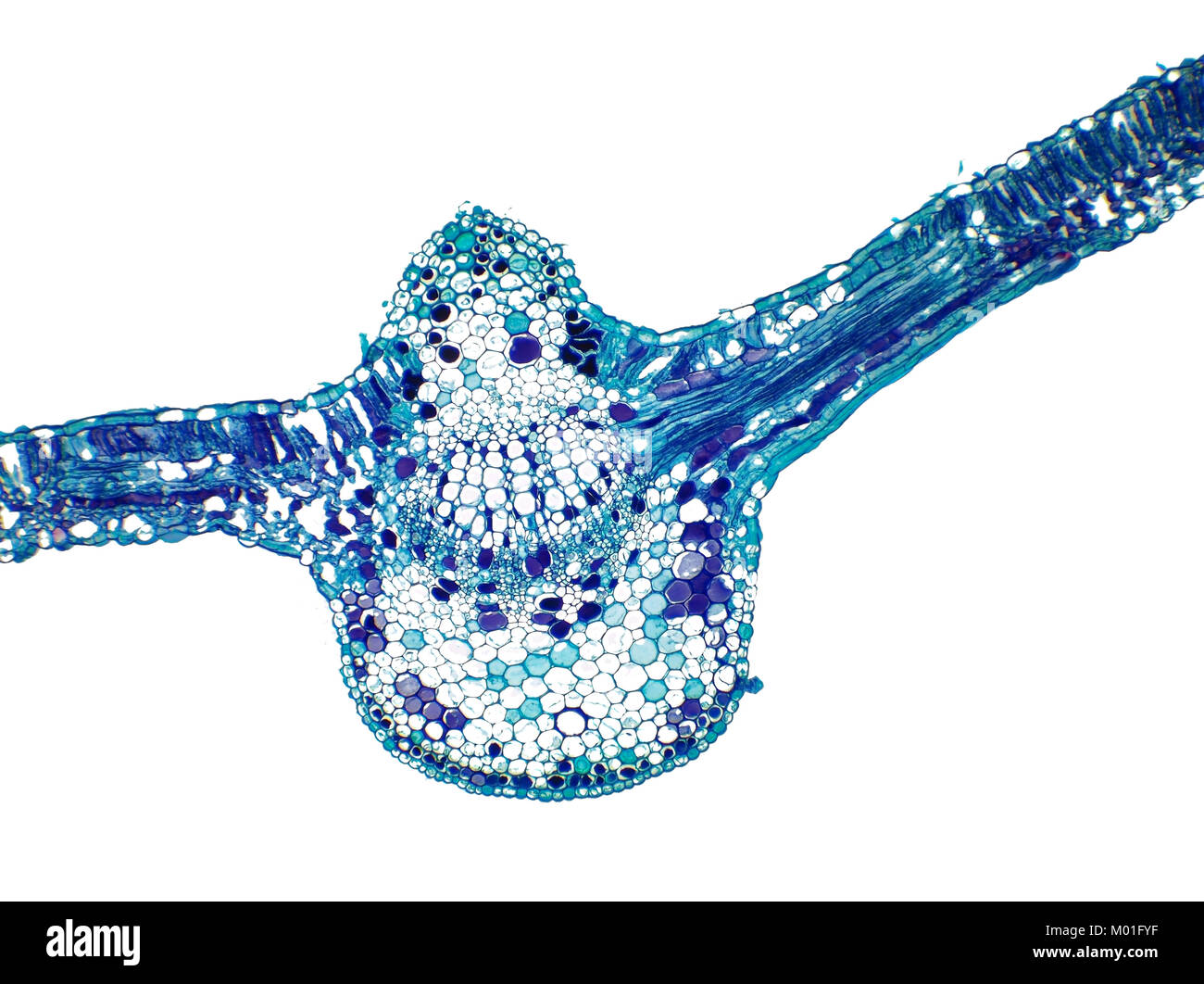 Bright field light micrograph of a cotton plant leaf, pictured area is 1.7mm wide Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/stock-photo-bright-field-light-micrograph-of-a-cotton-plant-leaf-pictured-area-172138163.html
Bright field light micrograph of a cotton plant leaf, pictured area is 1.7mm wide Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/stock-photo-bright-field-light-micrograph-of-a-cotton-plant-leaf-pictured-area-172138163.htmlRMM01FYF–Bright field light micrograph of a cotton plant leaf, pictured area is 1.7mm wide
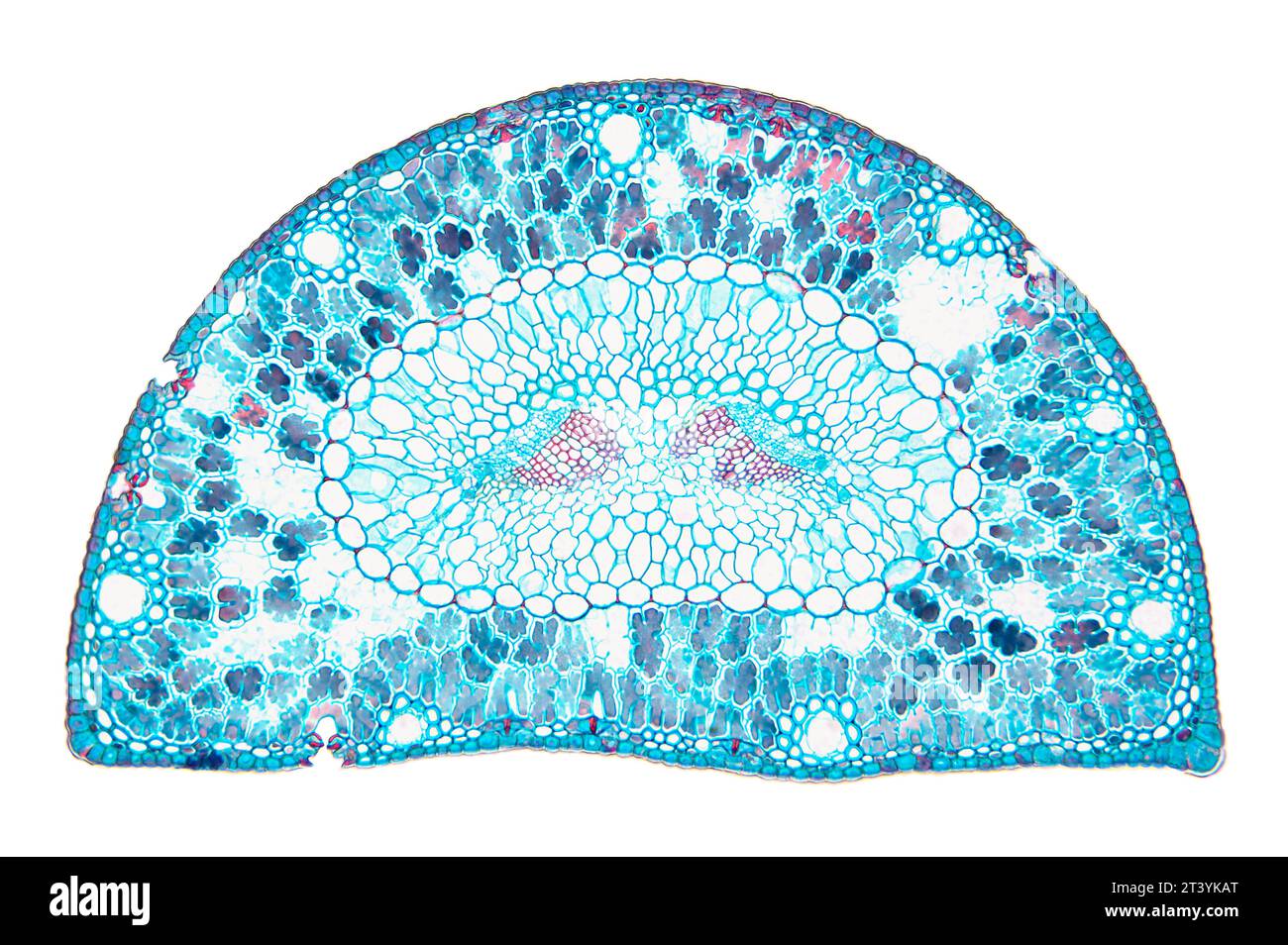
 Microscopic view of Mallow (Malva sp.) leaf cross section. Brightfield illumination. Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/microscopic-view-of-mallow-malva-sp-leaf-cross-section-brightfield-illumination-image210691107.html
Microscopic view of Mallow (Malva sp.) leaf cross section. Brightfield illumination. Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/microscopic-view-of-mallow-malva-sp-leaf-cross-section-brightfield-illumination-image210691107.htmlRFP6NPJB–Microscopic view of Mallow (Malva sp.) leaf cross section. Brightfield illumination.
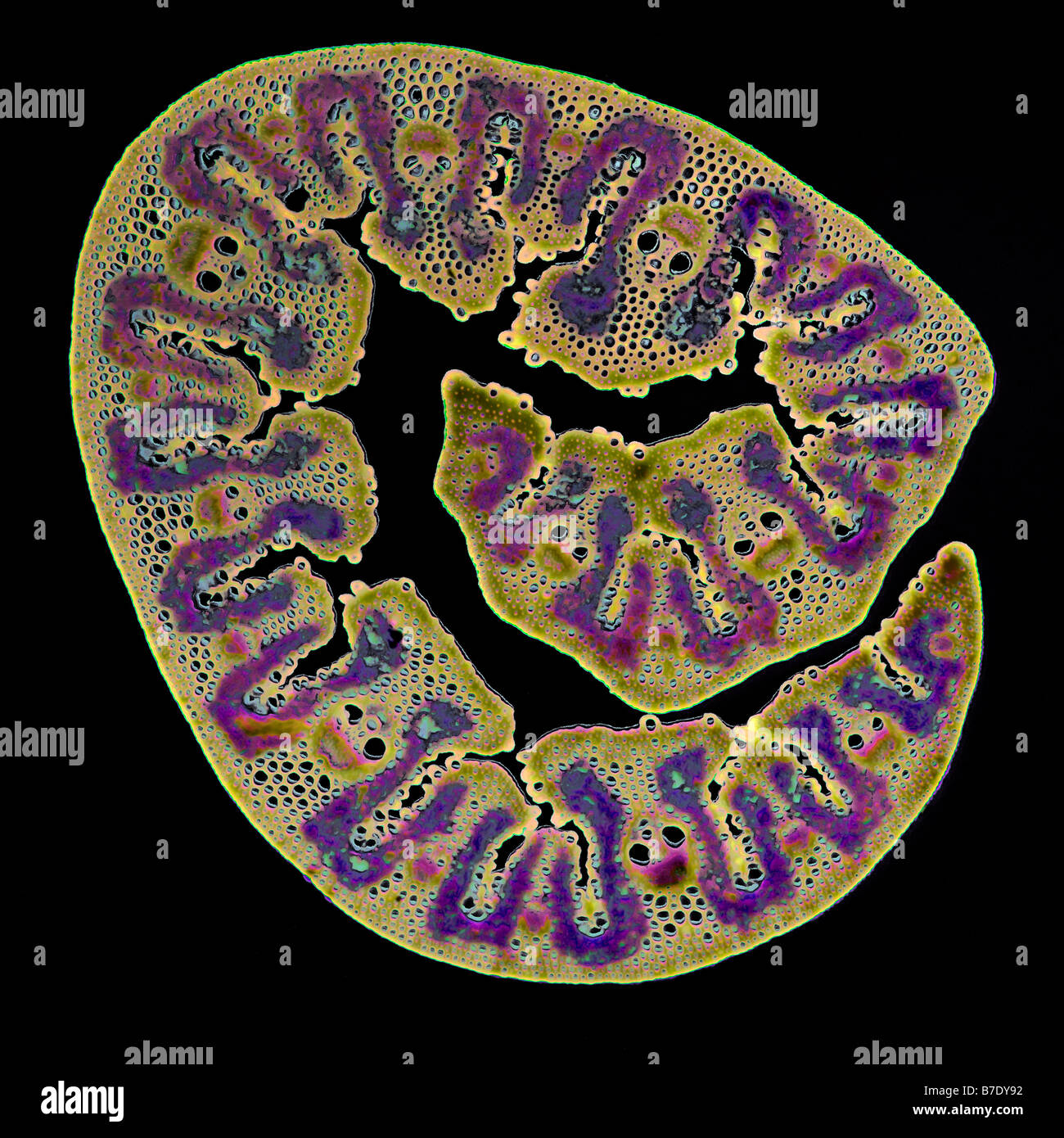 BEACH GRASS LEAF AMMOPHILA Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/stock-photo-beach-grass-leaf-ammophila-21819758.html
BEACH GRASS LEAF AMMOPHILA Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/stock-photo-beach-grass-leaf-ammophila-21819758.htmlRMB7DY92–BEACH GRASS LEAF AMMOPHILA
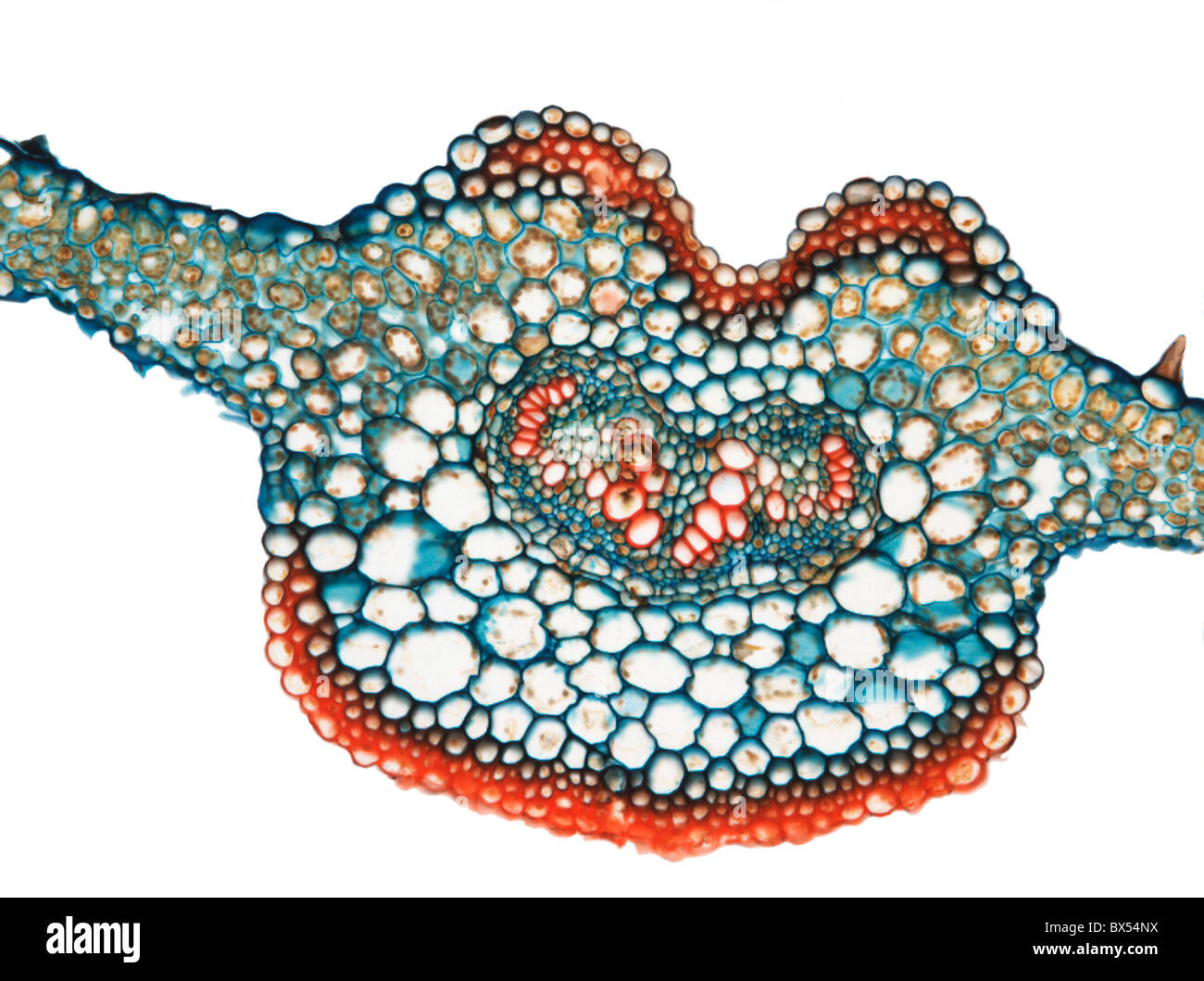
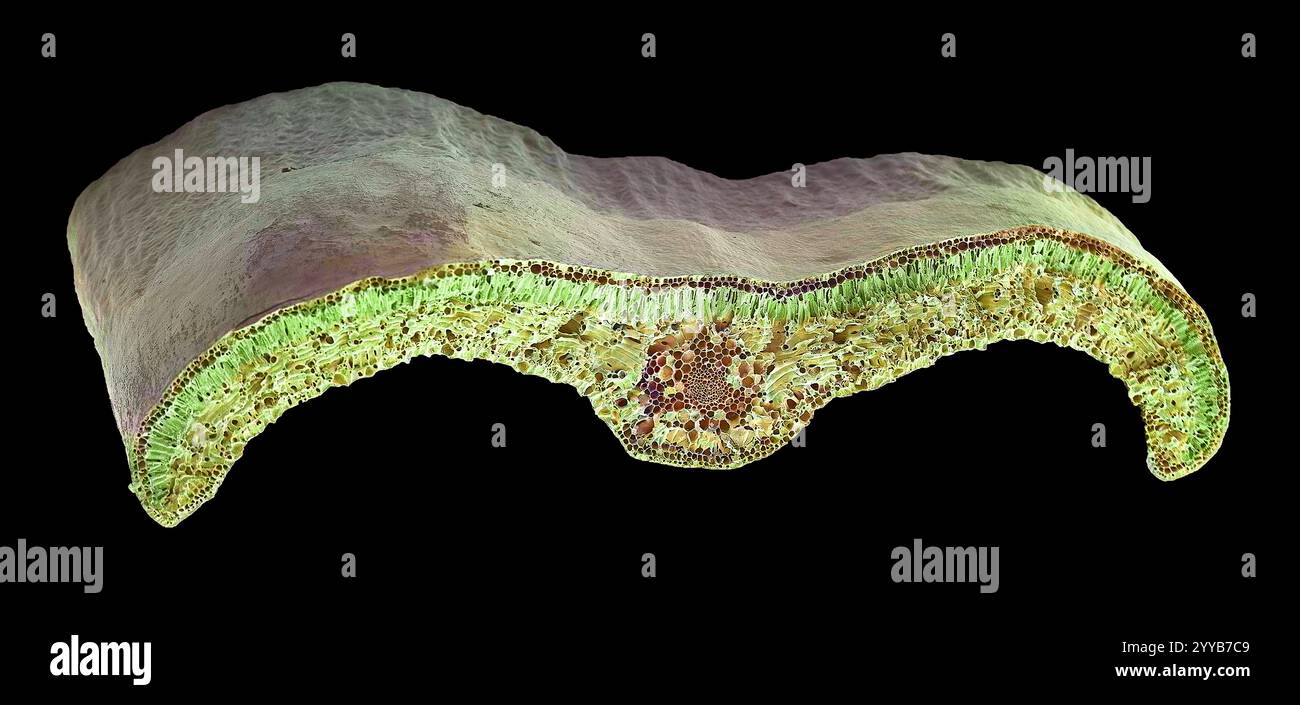 Box leaf. Coloured scanning electron micrograph (SEM) of a section through a leaf from the Common Box (Buxus sempervirens). The midrib (midvein) is the continuation of a leaf's stem along the centre of the leaf. At centre is a vascular bundle, which consists of xylem and phloem tissues. Xylem transports water and mineral nutrients from the roots throughout the plant and phloem transports carbohydrate and hormones around the plant. The surface (epidermis) of the leaf is covered in a waxy cuticle (top) that helps to prevent water loss. Magnification: x200 when printed 10 centimetres wide. Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/box-leaf-coloured-scanning-electron-micrograph-sem-of-a-section-through-a-leaf-from-the-common-box-buxus-sempervirens-the-midrib-midvein-is-the-continuation-of-a-leafs-stem-along-the-centre-of-the-leaf-at-centre-is-a-vascular-bundle-which-consists-of-xylem-and-phloem-tissues-xylem-transports-water-and-mineral-nutrients-from-the-roots-throughout-the-plant-and-phloem-transports-carbohydrate-and-hormones-around-the-plant-the-surface-epidermis-of-the-leaf-is-covered-in-a-waxy-cuticle-top-that-helps-to-prevent-water-loss-magnification-x200-when-printed-10-centimetres-wide-image636416265.html
Box leaf. Coloured scanning electron micrograph (SEM) of a section through a leaf from the Common Box (Buxus sempervirens). The midrib (midvein) is the continuation of a leaf's stem along the centre of the leaf. At centre is a vascular bundle, which consists of xylem and phloem tissues. Xylem transports water and mineral nutrients from the roots throughout the plant and phloem transports carbohydrate and hormones around the plant. The surface (epidermis) of the leaf is covered in a waxy cuticle (top) that helps to prevent water loss. Magnification: x200 when printed 10 centimetres wide. Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/box-leaf-coloured-scanning-electron-micrograph-sem-of-a-section-through-a-leaf-from-the-common-box-buxus-sempervirens-the-midrib-midvein-is-the-continuation-of-a-leafs-stem-along-the-centre-of-the-leaf-at-centre-is-a-vascular-bundle-which-consists-of-xylem-and-phloem-tissues-xylem-transports-water-and-mineral-nutrients-from-the-roots-throughout-the-plant-and-phloem-transports-carbohydrate-and-hormones-around-the-plant-the-surface-epidermis-of-the-leaf-is-covered-in-a-waxy-cuticle-top-that-helps-to-prevent-water-loss-magnification-x200-when-printed-10-centimetres-wide-image636416265.htmlRF2YYB7C9–Box leaf. Coloured scanning electron micrograph (SEM) of a section through a leaf from the Common Box (Buxus sempervirens). The midrib (midvein) is the continuation of a leaf's stem along the centre of the leaf. At centre is a vascular bundle, which consists of xylem and phloem tissues. Xylem transports water and mineral nutrients from the roots throughout the plant and phloem transports carbohydrate and hormones around the plant. The surface (epidermis) of the leaf is covered in a waxy cuticle (top) that helps to prevent water loss. Magnification: x200 when printed 10 centimetres wide.
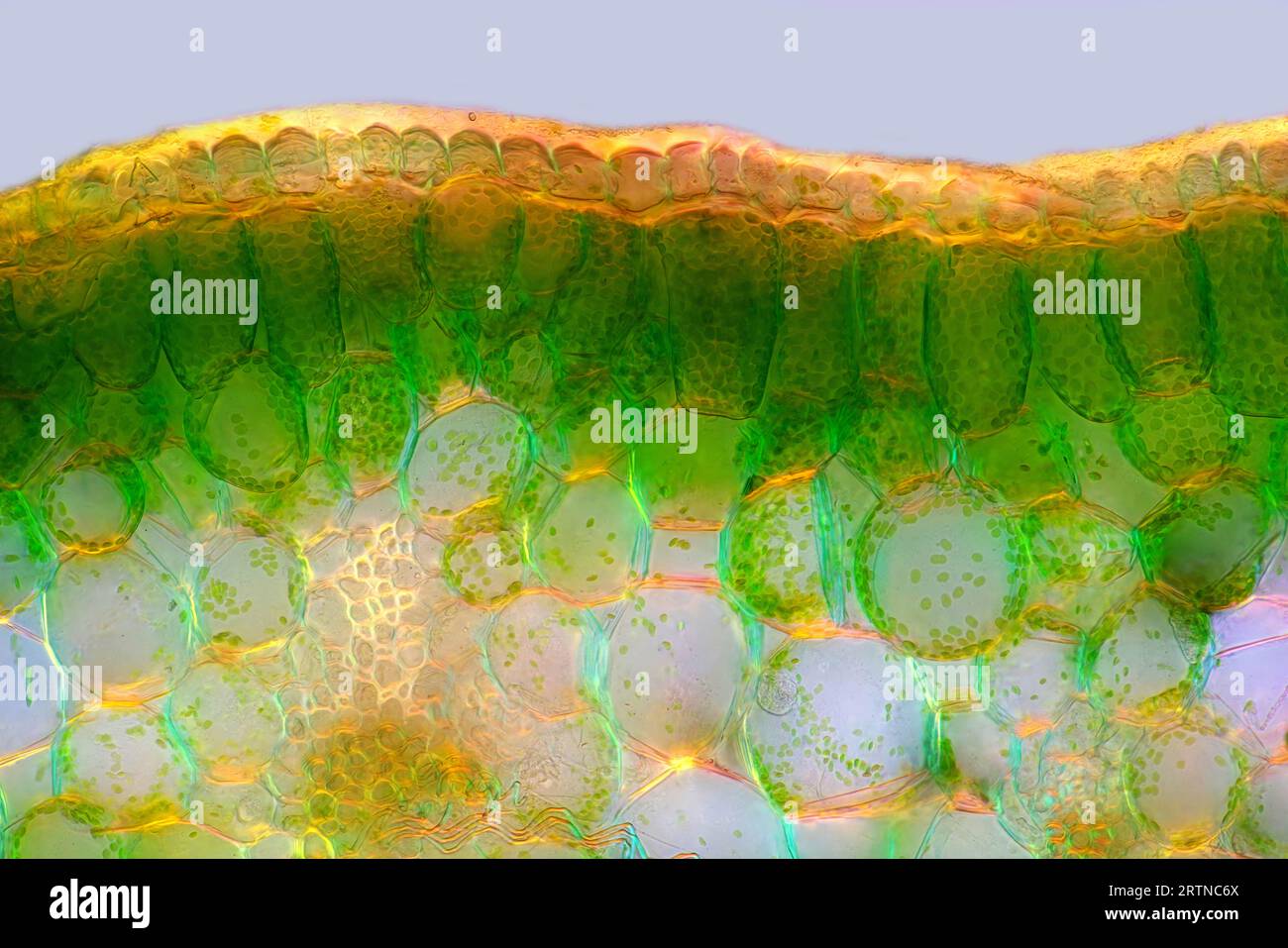 The image presents palisade mesophyll in hyacinthus leaf (transversal cross-section) photographed through the microscope in polarized light at a magn Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/the-image-presents-palisade-mesophyll-in-hyacinthus-leaf-transversal-cross-section-photographed-through-the-microscope-in-polarized-light-at-a-magn-image565954114.html
The image presents palisade mesophyll in hyacinthus leaf (transversal cross-section) photographed through the microscope in polarized light at a magn Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/the-image-presents-palisade-mesophyll-in-hyacinthus-leaf-transversal-cross-section-photographed-through-the-microscope-in-polarized-light-at-a-magn-image565954114.htmlRM2RTNC6X–The image presents palisade mesophyll in hyacinthus leaf (transversal cross-section) photographed through the microscope in polarized light at a magn
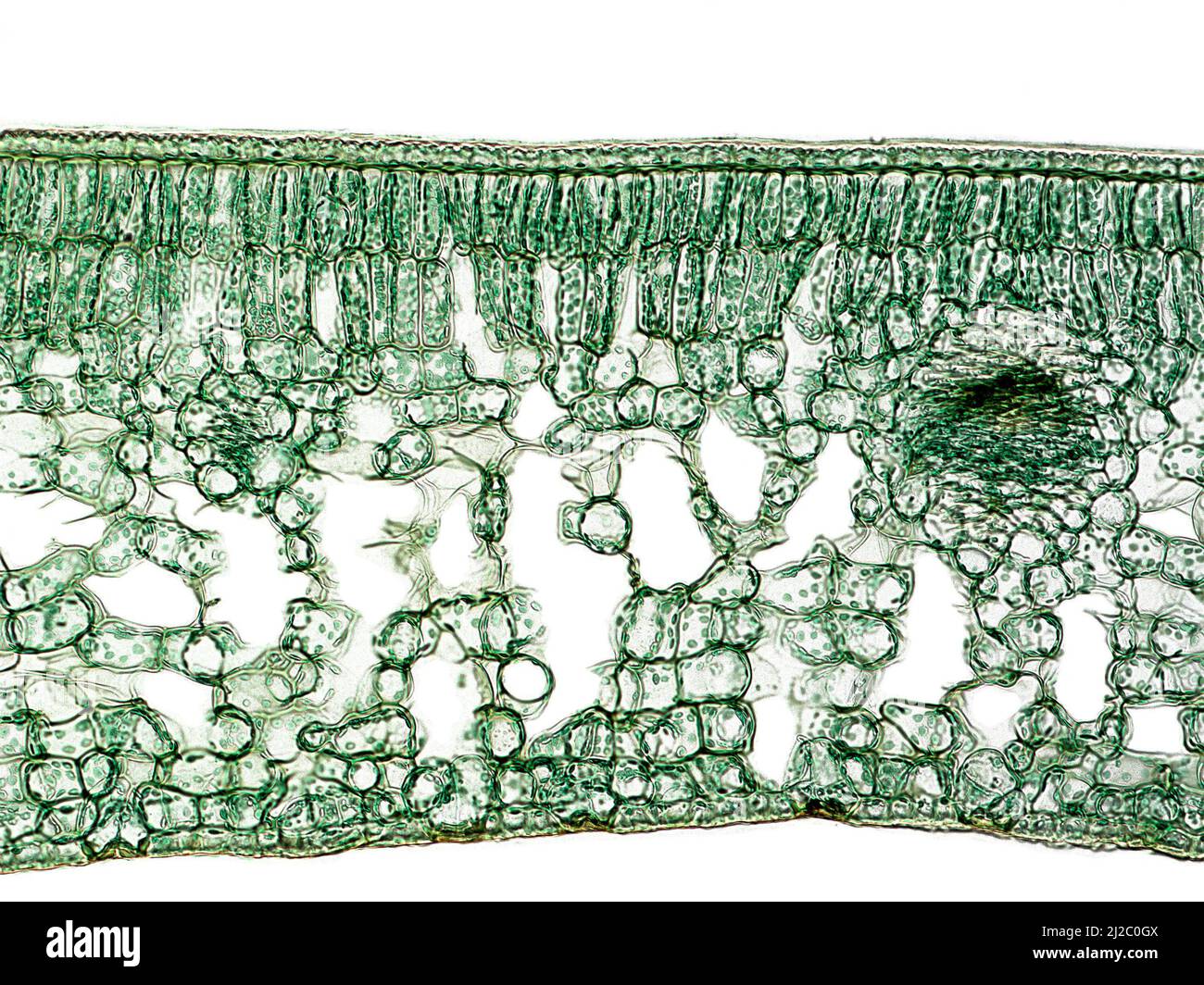 Cross section of a Camellia leaf, that show their general internal structure (cuticle, palisade parenchyma, spongy parenchyma, vascular bundles). Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/cross-section-of-a-camellia-leaf-that-show-their-general-internal-structure-cuticle-palisade-parenchyma-spongy-parenchyma-vascular-bundles-image466173146.html
Cross section of a Camellia leaf, that show their general internal structure (cuticle, palisade parenchyma, spongy parenchyma, vascular bundles). Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/cross-section-of-a-camellia-leaf-that-show-their-general-internal-structure-cuticle-palisade-parenchyma-spongy-parenchyma-vascular-bundles-image466173146.htmlRM2J2C0GX–Cross section of a Camellia leaf, that show their general internal structure (cuticle, palisade parenchyma, spongy parenchyma, vascular bundles).
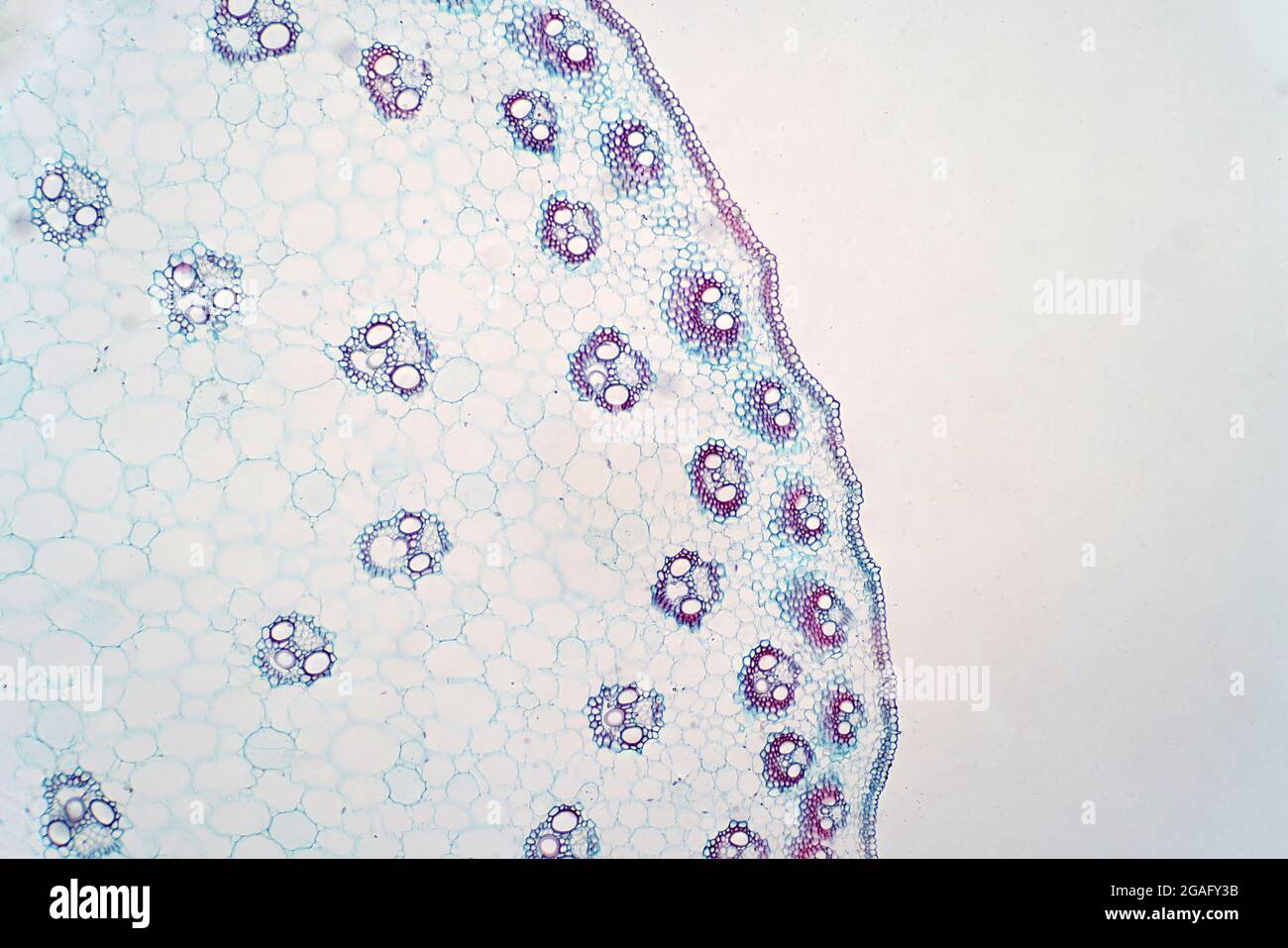
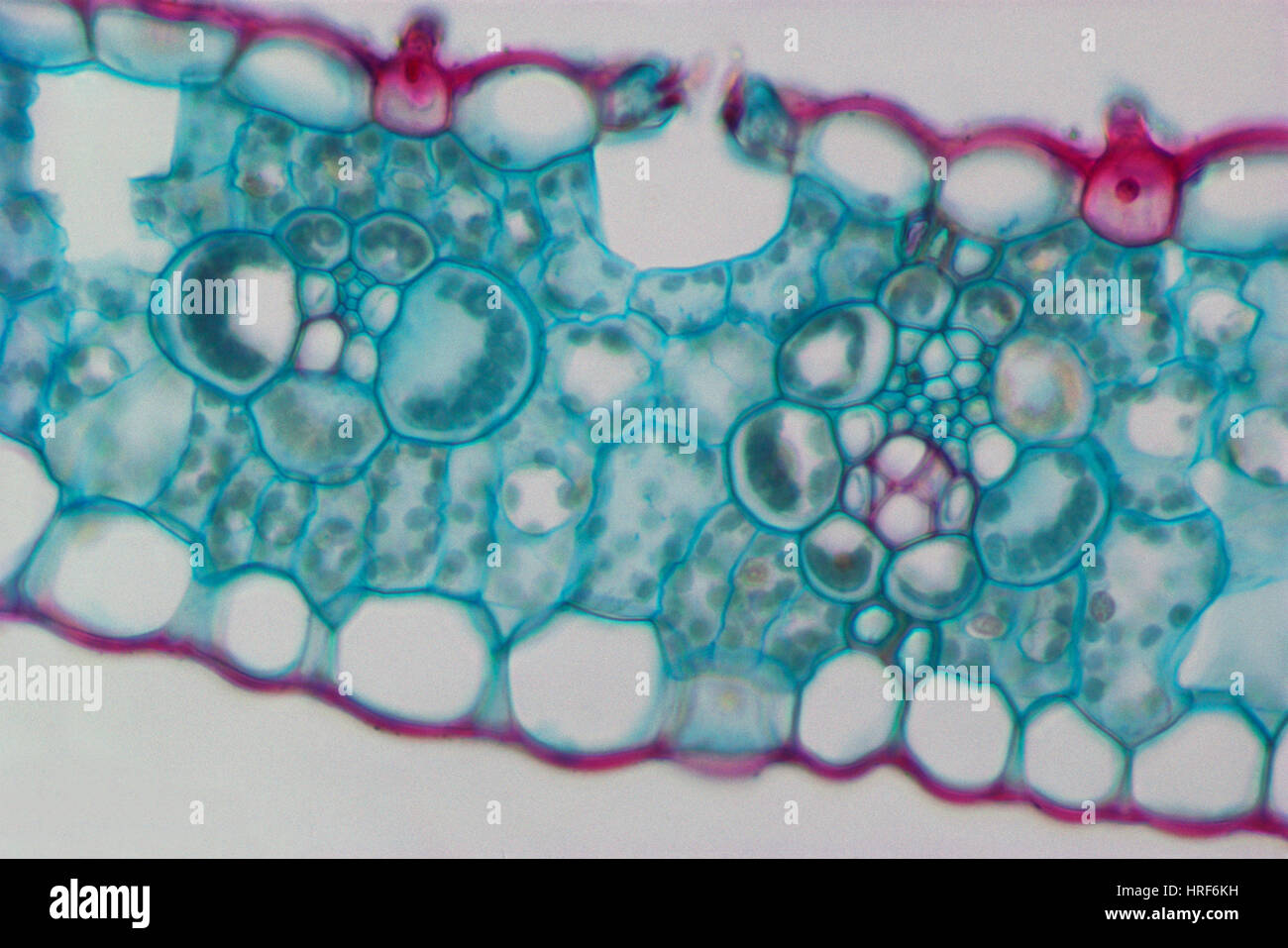 Corn Leaf Vascular Bundles and Sheaths Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/stock-photo-corn-leaf-vascular-bundles-and-sheaths-134944197.html
Corn Leaf Vascular Bundles and Sheaths Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/stock-photo-corn-leaf-vascular-bundles-and-sheaths-134944197.htmlRMHRF6KH–Corn Leaf Vascular Bundles and Sheaths
 The image presents read leaf in transversal cross-section, photographed through the microscope in polarized light at a magnification of 100X Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/the-image-presents-read-leaf-in-transversal-cross-section-photographed-through-the-microscope-in-polarized-light-at-a-magnification-of-100x-image564575503.html
The image presents read leaf in transversal cross-section, photographed through the microscope in polarized light at a magnification of 100X Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/the-image-presents-read-leaf-in-transversal-cross-section-photographed-through-the-microscope-in-polarized-light-at-a-magnification-of-100x-image564575503.htmlRM2RPEHPR–The image presents read leaf in transversal cross-section, photographed through the microscope in polarized light at a magnification of 100X
 Leaf section micrograph Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/leaf-section-micrograph-image362245008.html
Leaf section micrograph Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/leaf-section-micrograph-image362245008.htmlRM2C19K5M–Leaf section micrograph
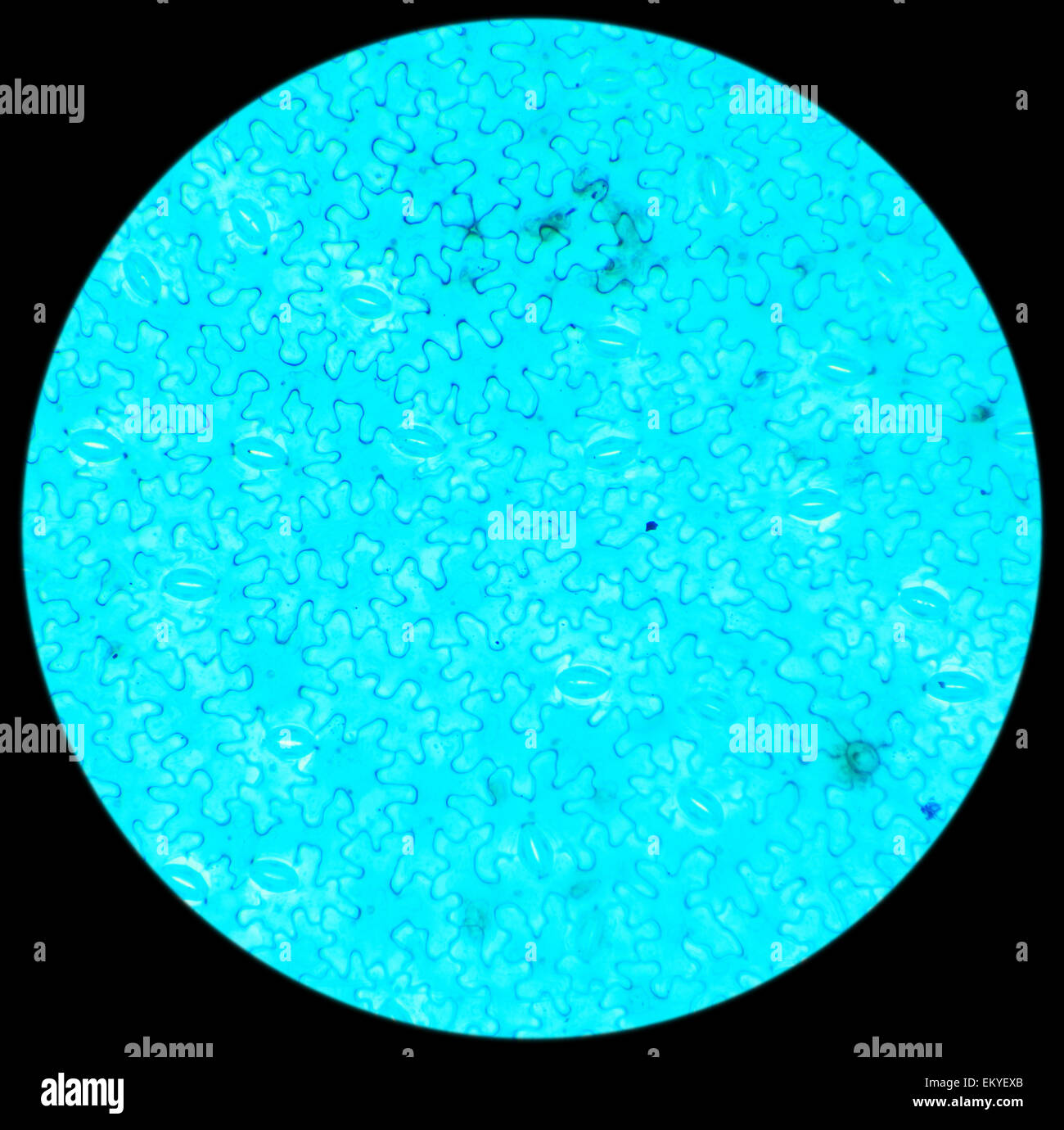 Vicia Dicot leaf under a microscope (Vicia Dicot leaf W.M.), 40x Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/stock-photo-vicia-dicot-leaf-under-a-microscope-vicia-dicot-leaf-wm-40x-81124355.html
Vicia Dicot leaf under a microscope (Vicia Dicot leaf W.M.), 40x Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/stock-photo-vicia-dicot-leaf-under-a-microscope-vicia-dicot-leaf-wm-40x-81124355.htmlRFEKYEXB–Vicia Dicot leaf under a microscope (Vicia Dicot leaf W.M.), 40x
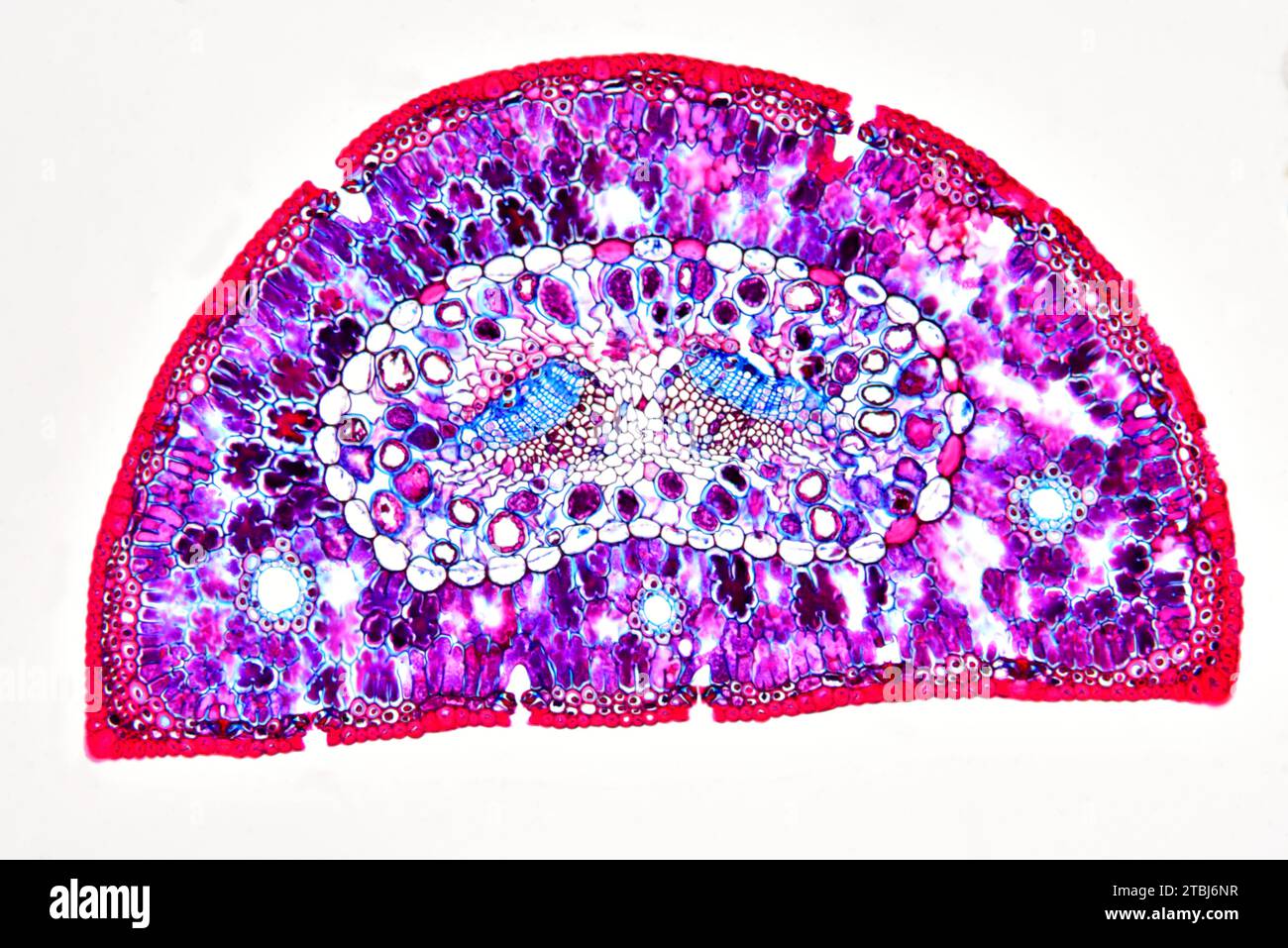 Leaf cross section of pine (Pinus sp.) showing endodermis, epidermis, hypodermis, mesophyll, resin canals, stomata, vascular bundle, phloem and xylem. Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/leaf-cross-section-of-pine-pinus-sp-showing-endodermis-epidermis-hypodermis-mesophyll-resin-canals-stomata-vascular-bundle-phloem-and-xylem-image575103811.html
Leaf cross section of pine (Pinus sp.) showing endodermis, epidermis, hypodermis, mesophyll, resin canals, stomata, vascular bundle, phloem and xylem. Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/leaf-cross-section-of-pine-pinus-sp-showing-endodermis-epidermis-hypodermis-mesophyll-resin-canals-stomata-vascular-bundle-phloem-and-xylem-image575103811.htmlRF2TBJ6NR–Leaf cross section of pine (Pinus sp.) showing endodermis, epidermis, hypodermis, mesophyll, resin canals, stomata, vascular bundle, phloem and xylem.
 science botany micrograph plant arabidopsis thaliana root tissue micro Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/stock-photo-science-botany-micrograph-plant-arabidopsis-thaliana-root-tissue-micro-50315336.html
science botany micrograph plant arabidopsis thaliana root tissue micro Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/stock-photo-science-botany-micrograph-plant-arabidopsis-thaliana-root-tissue-micro-50315336.htmlRFCWT1M8–science botany micrograph plant arabidopsis thaliana root tissue micro
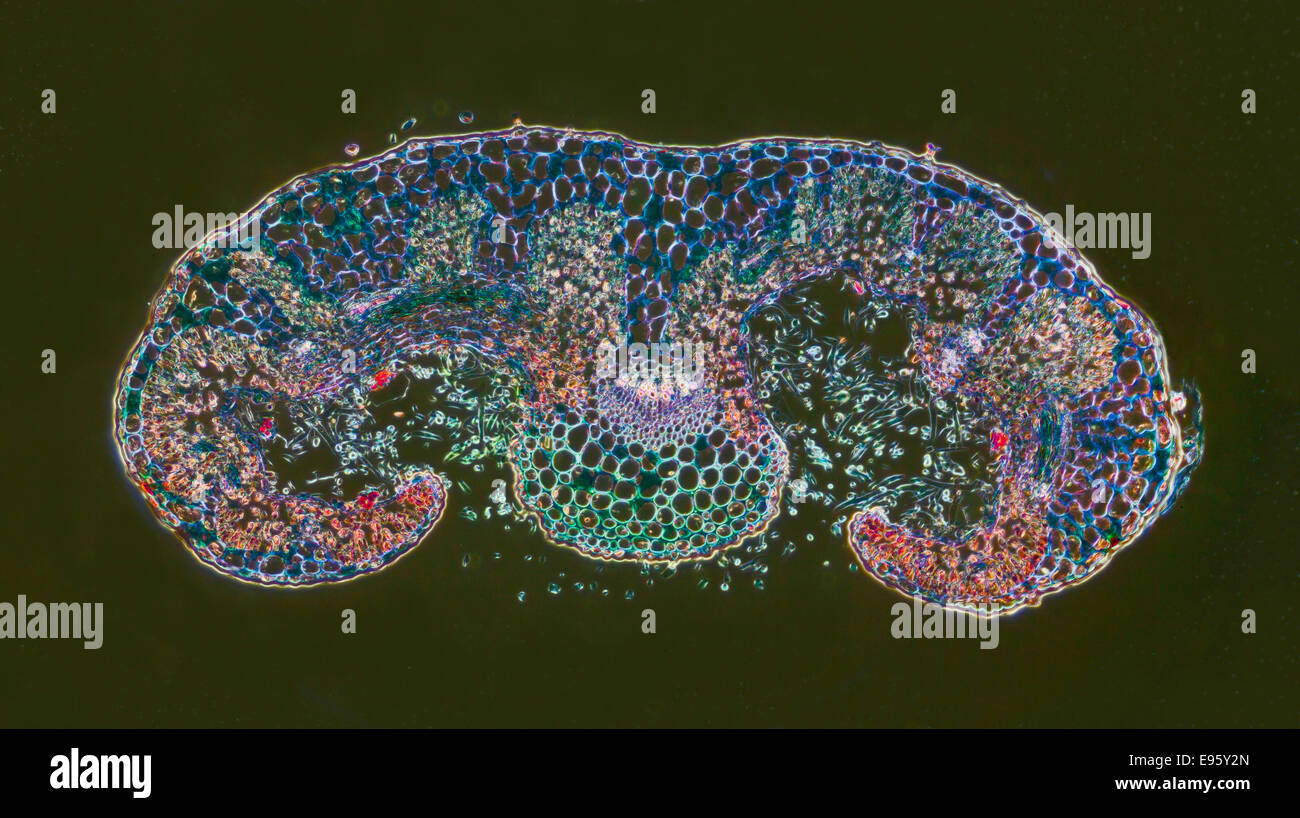 Rosemary, Rosmarinus officinalis, leaf TS, darkfield photomicrograph, stained section. Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/stock-photo-rosemary-rosmarinus-officinalis-leaf-ts-darkfield-photomicrograph-74504381.html
Rosemary, Rosmarinus officinalis, leaf TS, darkfield photomicrograph, stained section. Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/stock-photo-rosemary-rosmarinus-officinalis-leaf-ts-darkfield-photomicrograph-74504381.htmlRME95Y2N–Rosemary, Rosmarinus officinalis, leaf TS, darkfield photomicrograph, stained section.
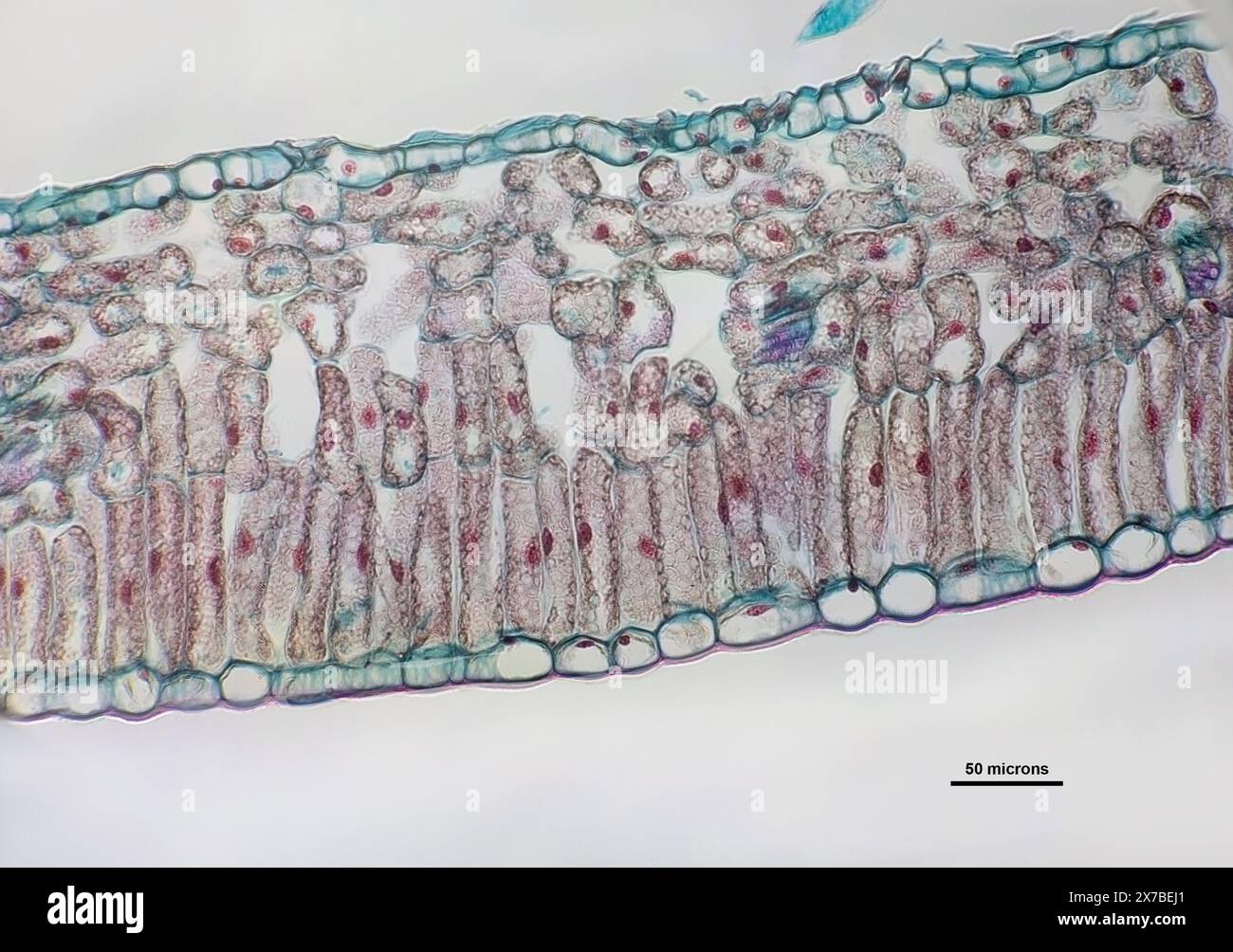 Privet leaf, transverse section under a microscope at 40 times magnification Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/privet-leaf-transverse-section-under-a-microscope-at-40-times-magnification-image606918425.html
Privet leaf, transverse section under a microscope at 40 times magnification Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/privet-leaf-transverse-section-under-a-microscope-at-40-times-magnification-image606918425.htmlRF2X7BEJ1–Privet leaf, transverse section under a microscope at 40 times magnification
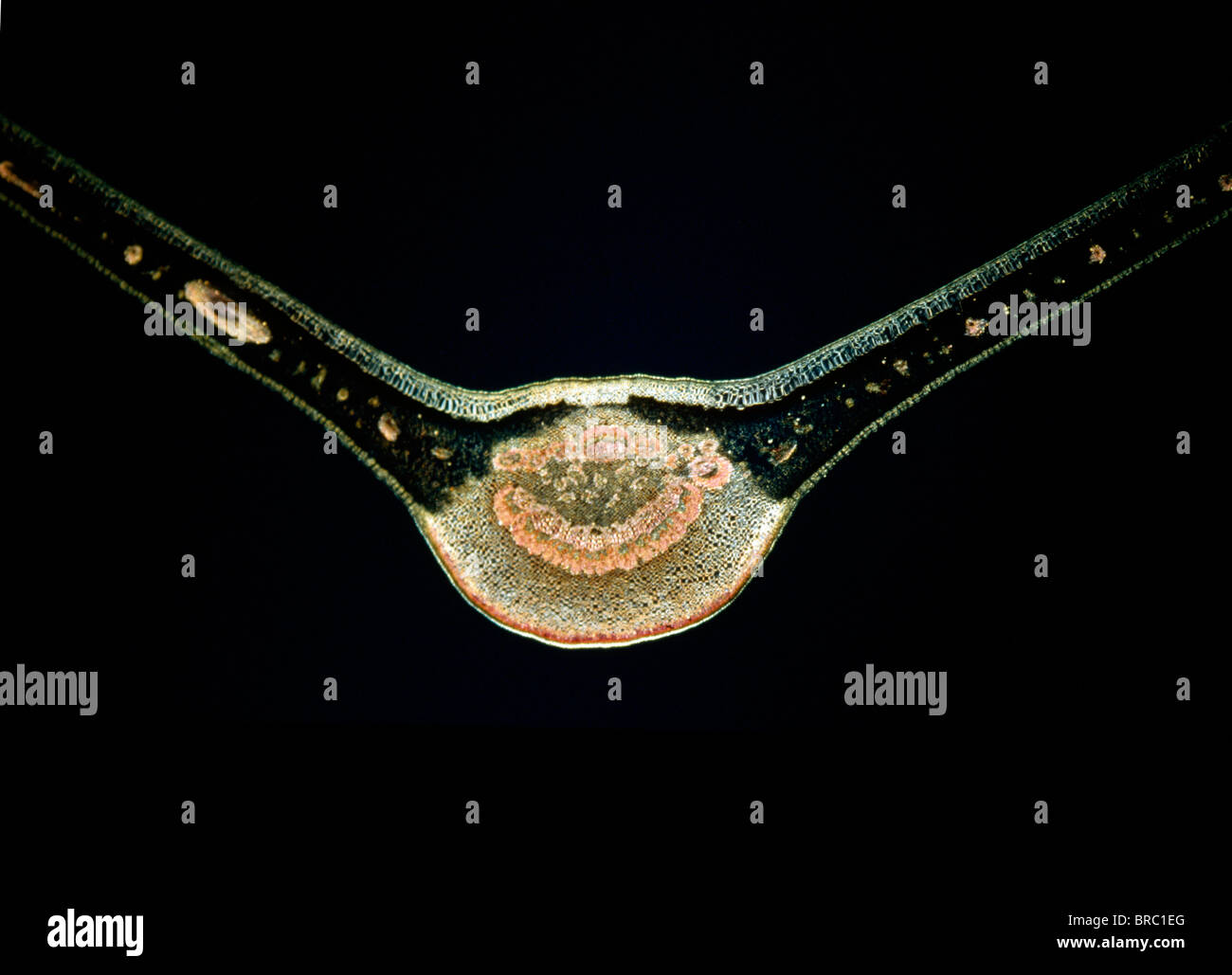 Light Micrograph (LM) of a transverse section of a fig leaf, magnification x 15 Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/stock-photo-light-micrograph-lm-of-a-transverse-section-of-a-fig-leaf-magnification-31612072.html
Light Micrograph (LM) of a transverse section of a fig leaf, magnification x 15 Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/stock-photo-light-micrograph-lm-of-a-transverse-section-of-a-fig-leaf-magnification-31612072.htmlRMBRC1EG–Light Micrograph (LM) of a transverse section of a fig leaf, magnification x 15
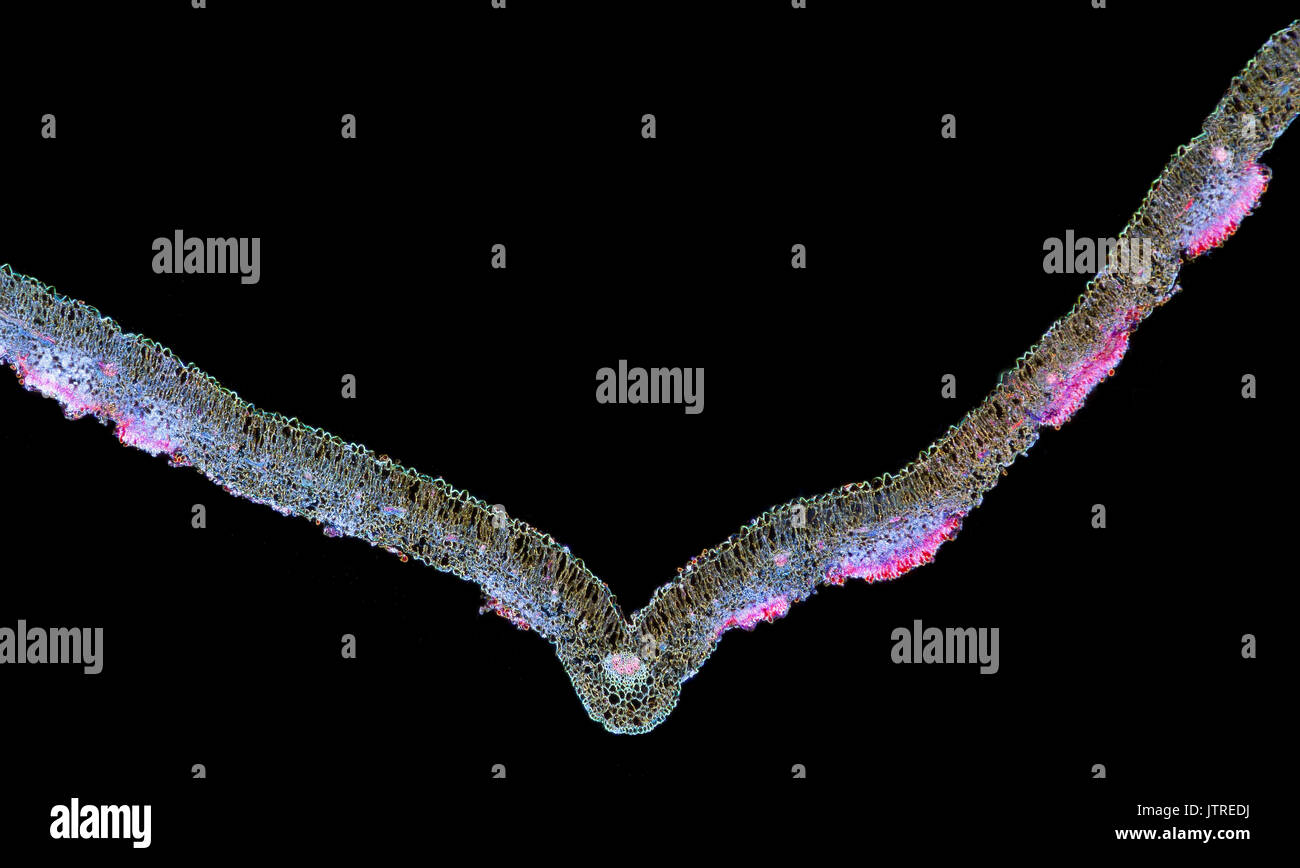 Snapdragon rust, Puccinia antirrhini fungus on antirrhinum leaf, darkfield photomicrograph, TS Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/snapdragon-rust-puccinia-antirrhini-fungus-on-antirrhinum-leaf-darkfield-image152950942.html
Snapdragon rust, Puccinia antirrhini fungus on antirrhinum leaf, darkfield photomicrograph, TS Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/snapdragon-rust-puccinia-antirrhini-fungus-on-antirrhinum-leaf-darkfield-image152950942.htmlRMJTREDJ–Snapdragon rust, Puccinia antirrhini fungus on antirrhinum leaf, darkfield photomicrograph, TS
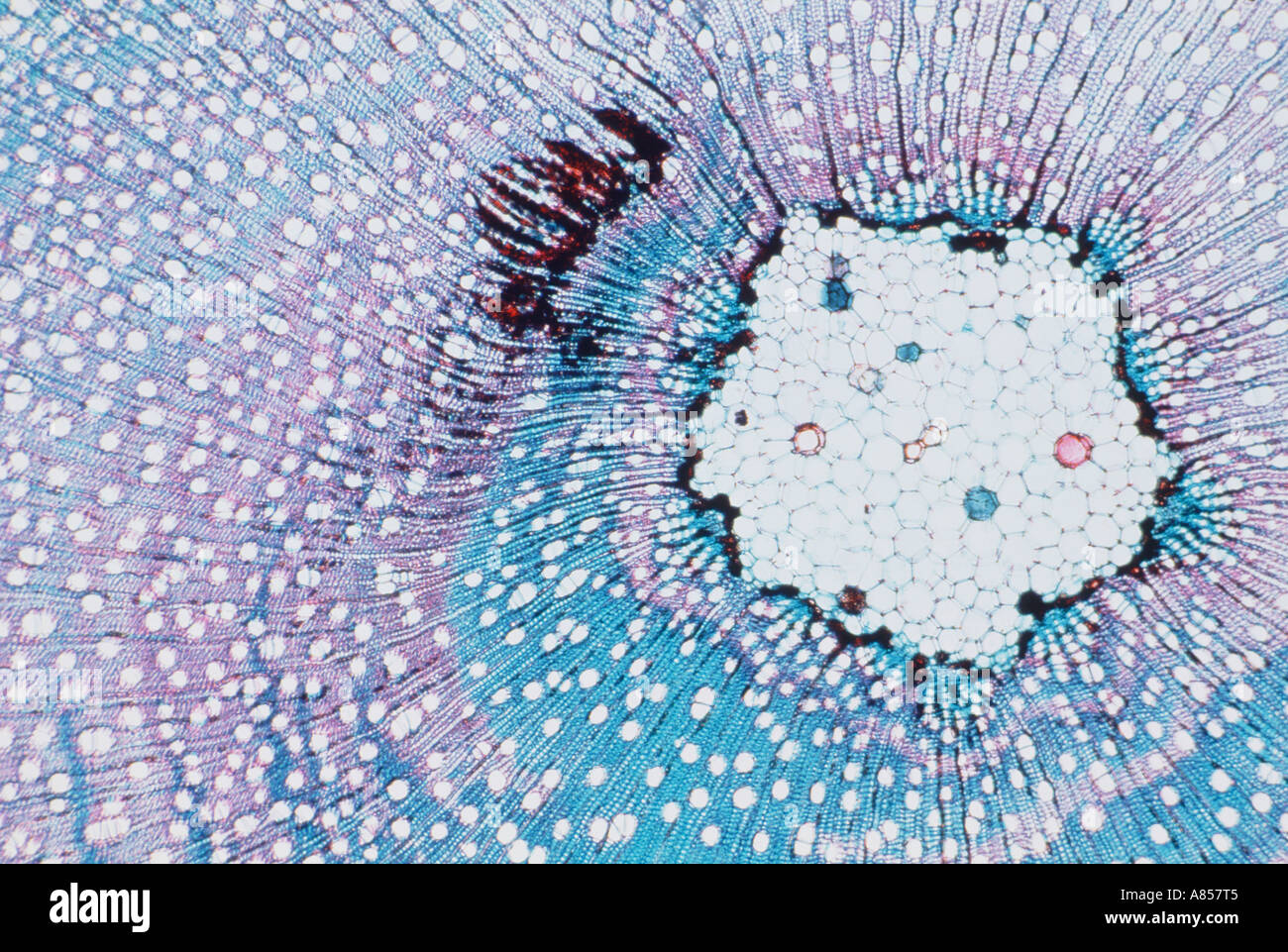
 Leaf micrograph Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/stock-photo-leaf-micrograph-80171364.html
Leaf micrograph Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/stock-photo-leaf-micrograph-80171364.htmlRFEJC3B0–Leaf micrograph
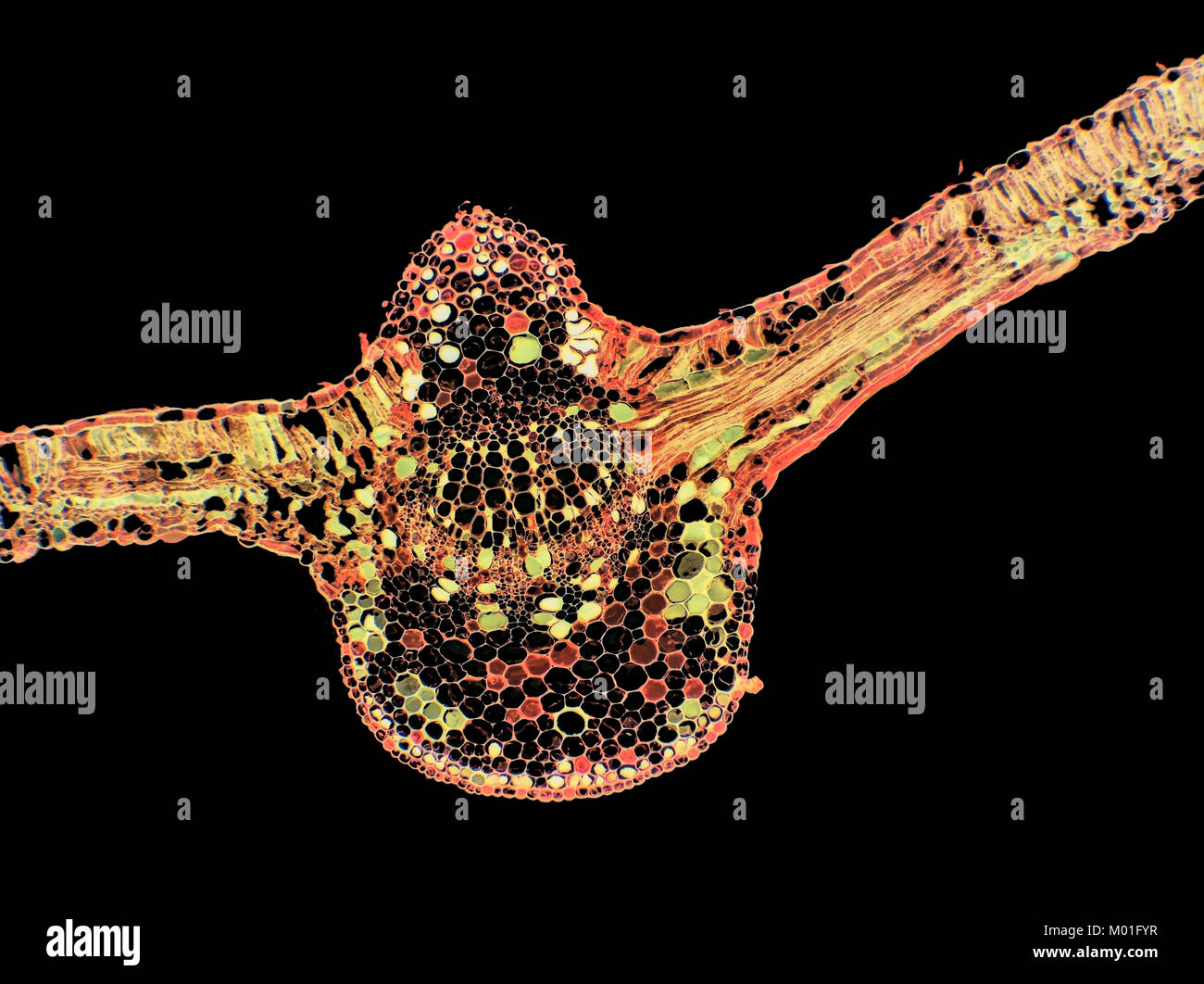
 Scanning Electron Micrograph (SEM) of willow tree leaf stem cross-section. Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/scanning-electron-micrograph-sem-of-willow-tree-leaf-stem-cross-section-image6852292.html
Scanning Electron Micrograph (SEM) of willow tree leaf stem cross-section. Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/scanning-electron-micrograph-sem-of-willow-tree-leaf-stem-cross-section-image6852292.htmlRMA857T5–Scanning Electron Micrograph (SEM) of willow tree leaf stem cross-section.
 Pine leaf micrograph Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/stock-photo-pine-leaf-micrograph-93552931.html
Pine leaf micrograph Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/stock-photo-pine-leaf-micrograph-93552931.htmlRFFC5KM3–Pine leaf micrograph
 Inverted bright field light micrograph of a cotton plant leaf, pictured area is 1.7mm wide Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/stock-photo-inverted-bright-field-light-micrograph-of-a-cotton-plant-leaf-pictured-172138171.html
Inverted bright field light micrograph of a cotton plant leaf, pictured area is 1.7mm wide Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/stock-photo-inverted-bright-field-light-micrograph-of-a-cotton-plant-leaf-pictured-172138171.htmlRMM01FYR–Inverted bright field light micrograph of a cotton plant leaf, pictured area is 1.7mm wide
 Microscopic view of a Showy stonecrop (Hylotelephium spectabile) leaf cross section. Polarized light, crossed polarizers. Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/microscopic-view-of-a-showy-stonecrop-hylotelephium-spectabile-leaf-cross-section-polarized-light-crossed-polarizers-image210691117.html
Microscopic view of a Showy stonecrop (Hylotelephium spectabile) leaf cross section. Polarized light, crossed polarizers. Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/microscopic-view-of-a-showy-stonecrop-hylotelephium-spectabile-leaf-cross-section-polarized-light-crossed-polarizers-image210691117.htmlRFP6NPJN–Microscopic view of a Showy stonecrop (Hylotelephium spectabile) leaf cross section. Polarized light, crossed polarizers.
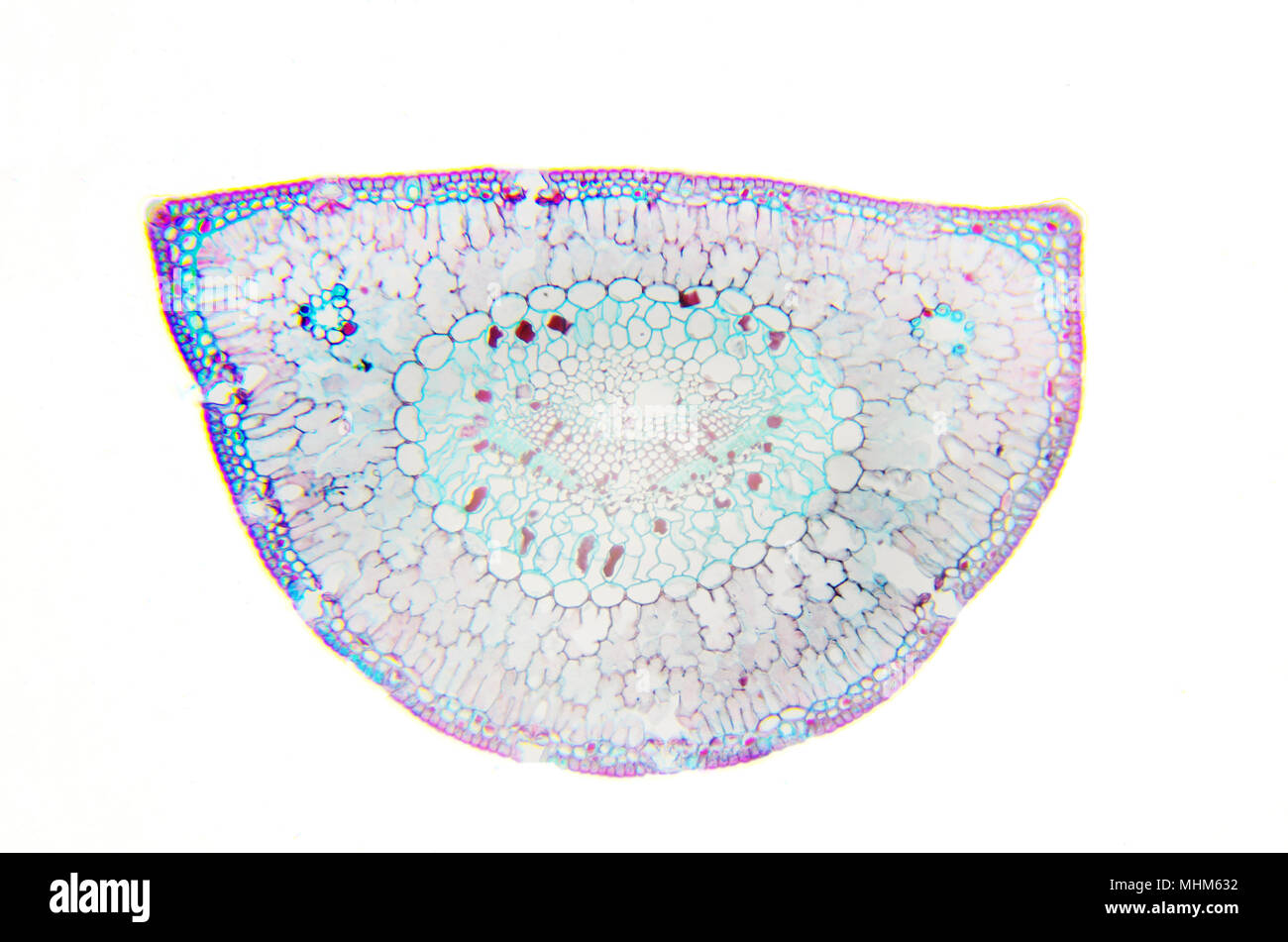 Microscopy Photography. Leaf of Pinus. Transversal Section. Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/microscopy-photography-leaf-of-pinus-transversal-section-image182996662.html
Microscopy Photography. Leaf of Pinus. Transversal Section. Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/microscopy-photography-leaf-of-pinus-transversal-section-image182996662.htmlRFMHM632–Microscopy Photography. Leaf of Pinus. Transversal Section.
 Box leaf. Coloured scanning electron micrograph (SEM) of a section through a leaf from the Common Box (Buxus sempervirens). The midrib (midvein) is the continuation of a leaf's stem along the centre of the leaf. At centre is a vascular bundle, which consists of xylem and phloem tissues. Xylem transports water and mineral nutrients from the roots throughout the plant and phloem transports carbohydrate and hormones around the plant. The surface (epidermis) of the leaf is covered in a waxy cuticle (top) that helps to prevent water loss. Magnification: x200 when printed 10 centimetres wide. Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/box-leaf-coloured-scanning-electron-micrograph-sem-of-a-section-through-a-leaf-from-the-common-box-buxus-sempervirens-the-midrib-midvein-is-the-continuation-of-a-leafs-stem-along-the-centre-of-the-leaf-at-centre-is-a-vascular-bundle-which-consists-of-xylem-and-phloem-tissues-xylem-transports-water-and-mineral-nutrients-from-the-roots-throughout-the-plant-and-phloem-transports-carbohydrate-and-hormones-around-the-plant-the-surface-epidermis-of-the-leaf-is-covered-in-a-waxy-cuticle-top-that-helps-to-prevent-water-loss-magnification-x200-when-printed-10-centimetres-wide-image636416299.html
Box leaf. Coloured scanning electron micrograph (SEM) of a section through a leaf from the Common Box (Buxus sempervirens). The midrib (midvein) is the continuation of a leaf's stem along the centre of the leaf. At centre is a vascular bundle, which consists of xylem and phloem tissues. Xylem transports water and mineral nutrients from the roots throughout the plant and phloem transports carbohydrate and hormones around the plant. The surface (epidermis) of the leaf is covered in a waxy cuticle (top) that helps to prevent water loss. Magnification: x200 when printed 10 centimetres wide. Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/box-leaf-coloured-scanning-electron-micrograph-sem-of-a-section-through-a-leaf-from-the-common-box-buxus-sempervirens-the-midrib-midvein-is-the-continuation-of-a-leafs-stem-along-the-centre-of-the-leaf-at-centre-is-a-vascular-bundle-which-consists-of-xylem-and-phloem-tissues-xylem-transports-water-and-mineral-nutrients-from-the-roots-throughout-the-plant-and-phloem-transports-carbohydrate-and-hormones-around-the-plant-the-surface-epidermis-of-the-leaf-is-covered-in-a-waxy-cuticle-top-that-helps-to-prevent-water-loss-magnification-x200-when-printed-10-centimetres-wide-image636416299.htmlRF2YYB7DF–Box leaf. Coloured scanning electron micrograph (SEM) of a section through a leaf from the Common Box (Buxus sempervirens). The midrib (midvein) is the continuation of a leaf's stem along the centre of the leaf. At centre is a vascular bundle, which consists of xylem and phloem tissues. Xylem transports water and mineral nutrients from the roots throughout the plant and phloem transports carbohydrate and hormones around the plant. The surface (epidermis) of the leaf is covered in a waxy cuticle (top) that helps to prevent water loss. Magnification: x200 when printed 10 centimetres wide.
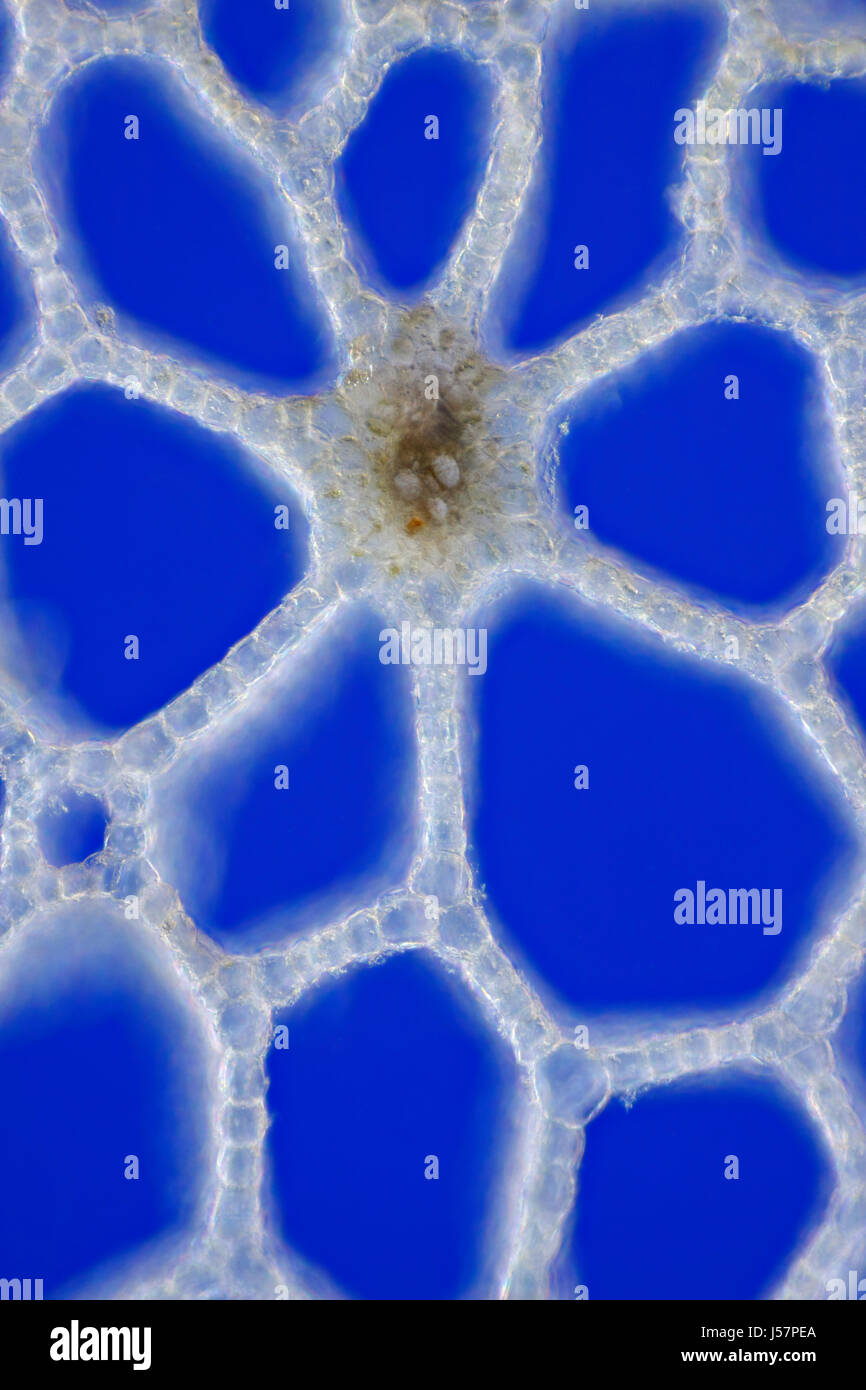 Marsh Calla (Calla palustris) leaf stem cross section. Rheinberg illumination. Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/stock-photo-marsh-calla-calla-palustris-leaf-stem-cross-section-rheinberg-illumination-140927538.html
Marsh Calla (Calla palustris) leaf stem cross section. Rheinberg illumination. Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/stock-photo-marsh-calla-calla-palustris-leaf-stem-cross-section-rheinberg-illumination-140927538.htmlRFJ57PEA–Marsh Calla (Calla palustris) leaf stem cross section. Rheinberg illumination.
 biological cell isolated on whithe background microscope 3D Illustration Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/biological-cell-isolated-on-whithe-background-microscope-3d-illustration-image208953290.html
biological cell isolated on whithe background microscope 3D Illustration Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/biological-cell-isolated-on-whithe-background-microscope-3d-illustration-image208953290.htmlRFP3XJ1E–biological cell isolated on whithe background microscope 3D Illustration
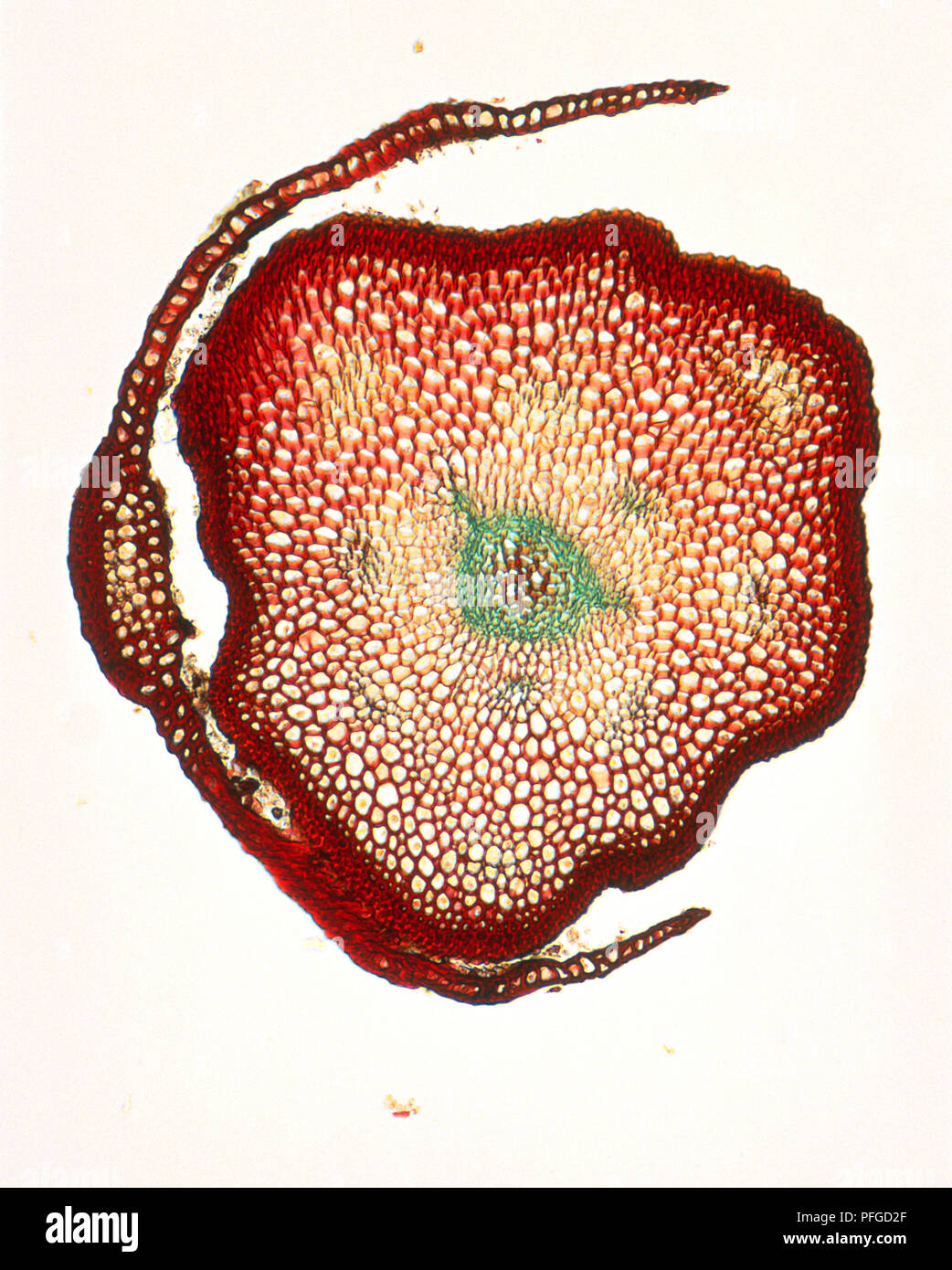 Micrograph of stem and leaf of Polytrichum commune (Common moss), cross-section Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/micrograph-of-stem-and-leaf-of-polytrichum-commune-common-moss-cross-section-image216105751.html
Micrograph of stem and leaf of Polytrichum commune (Common moss), cross-section Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/micrograph-of-stem-and-leaf-of-polytrichum-commune-common-moss-cross-section-image216105751.htmlRMPFGD2F–Micrograph of stem and leaf of Polytrichum commune (Common moss), cross-section
 The image presents carex sp. leaf in transversal cross-section, photographed through the microscope in polarized light at a magnification of 100X Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/the-image-presents-carex-sp-leaf-in-transversal-cross-section-photographed-through-the-microscope-in-polarized-light-at-a-magnification-of-100x-image564575213.html
The image presents carex sp. leaf in transversal cross-section, photographed through the microscope in polarized light at a magnification of 100X Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/the-image-presents-carex-sp-leaf-in-transversal-cross-section-photographed-through-the-microscope-in-polarized-light-at-a-magnification-of-100x-image564575213.htmlRM2RPEHCD–The image presents carex sp. leaf in transversal cross-section, photographed through the microscope in polarized light at a magnification of 100X
 Leaf micrograph Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/stock-photo-leaf-micrograph-103147644.html
Leaf micrograph Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/stock-photo-leaf-micrograph-103147644.htmlRMFYPNTC–Leaf micrograph
 Vicia Dicot leaf under a microscope (Vicia Dicot leaf W.M.), 400x Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/stock-photo-vicia-dicot-leaf-under-a-microscope-vicia-dicot-leaf-wm-400x-80958639.html
Vicia Dicot leaf under a microscope (Vicia Dicot leaf W.M.), 400x Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/stock-photo-vicia-dicot-leaf-under-a-microscope-vicia-dicot-leaf-wm-400x-80958639.htmlRFEKKYFY–Vicia Dicot leaf under a microscope (Vicia Dicot leaf W.M.), 400x
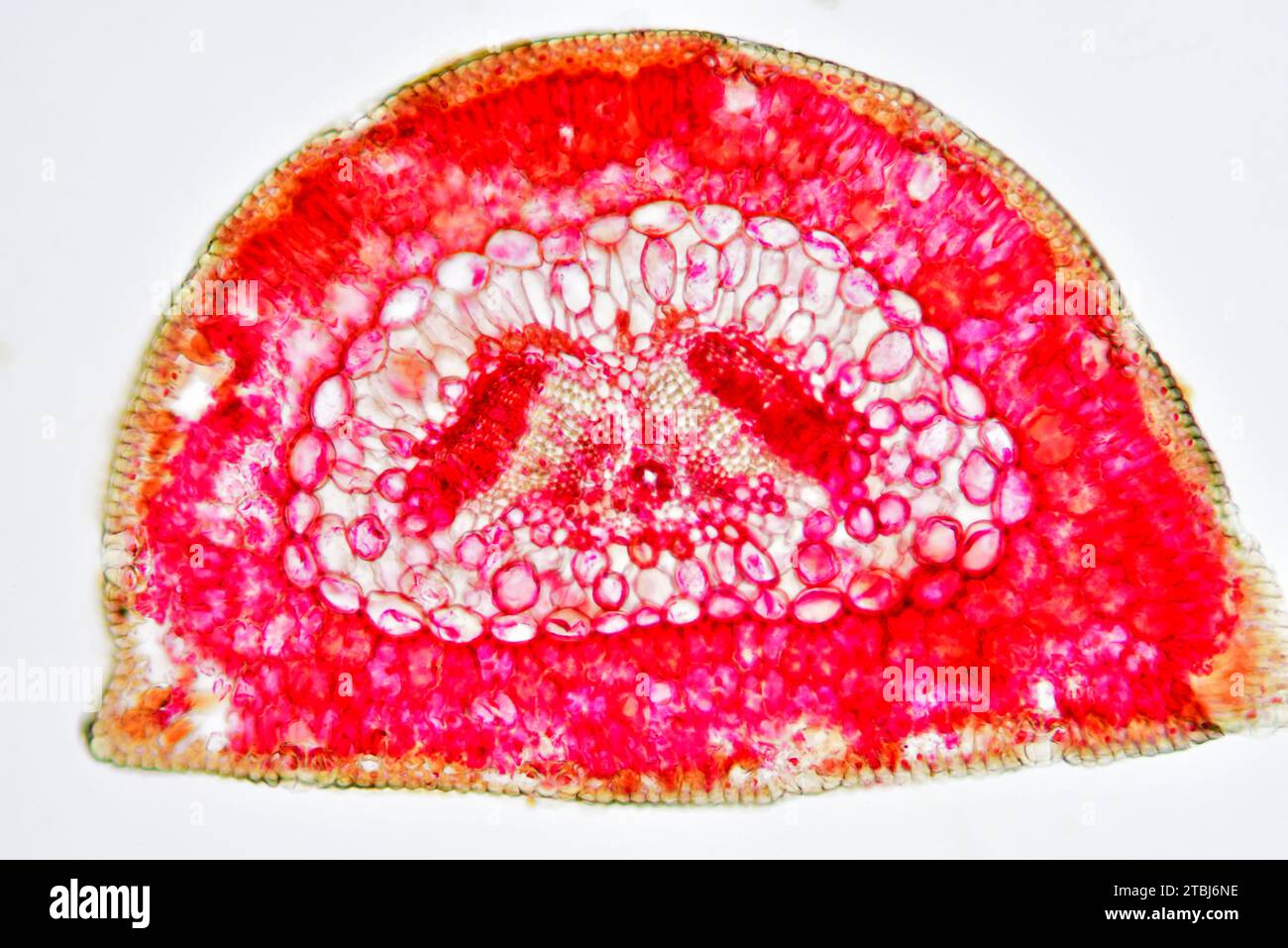 Leaf cross section of pine (Pinus sp.) showing endodermis, epidermis, hypodermis, mesophyll, resin canals, stomata, vascular bundle, phloem and xylem. Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/leaf-cross-section-of-pine-pinus-sp-showing-endodermis-epidermis-hypodermis-mesophyll-resin-canals-stomata-vascular-bundle-phloem-and-xylem-image575103802.html
Leaf cross section of pine (Pinus sp.) showing endodermis, epidermis, hypodermis, mesophyll, resin canals, stomata, vascular bundle, phloem and xylem. Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/leaf-cross-section-of-pine-pinus-sp-showing-endodermis-epidermis-hypodermis-mesophyll-resin-canals-stomata-vascular-bundle-phloem-and-xylem-image575103802.htmlRF2TBJ6NE–Leaf cross section of pine (Pinus sp.) showing endodermis, epidermis, hypodermis, mesophyll, resin canals, stomata, vascular bundle, phloem and xylem.
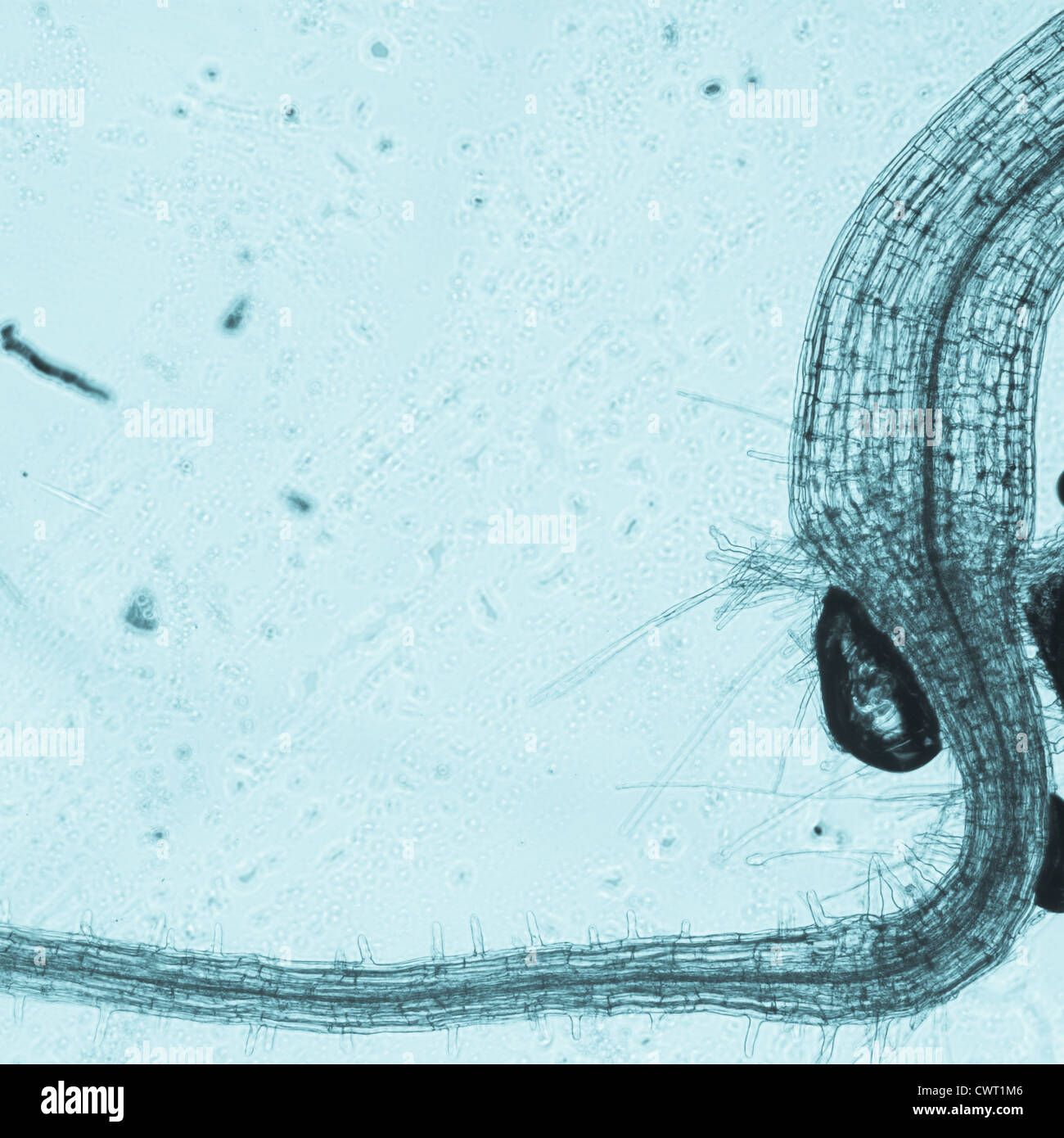 science botany micrograph plant arabidopsis thaliana root tissue micro Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/stock-photo-science-botany-micrograph-plant-arabidopsis-thaliana-root-tissue-micro-50315334.html
science botany micrograph plant arabidopsis thaliana root tissue micro Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/stock-photo-science-botany-micrograph-plant-arabidopsis-thaliana-root-tissue-micro-50315334.htmlRFCWT1M6–science botany micrograph plant arabidopsis thaliana root tissue micro
 biological cell isolated on whithe background microscope 3D Illustration Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/biological-cell-isolated-on-whithe-background-microscope-3d-illustration-image208953338.html
biological cell isolated on whithe background microscope 3D Illustration Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/biological-cell-isolated-on-whithe-background-microscope-3d-illustration-image208953338.htmlRFP3XJ36–biological cell isolated on whithe background microscope 3D Illustration
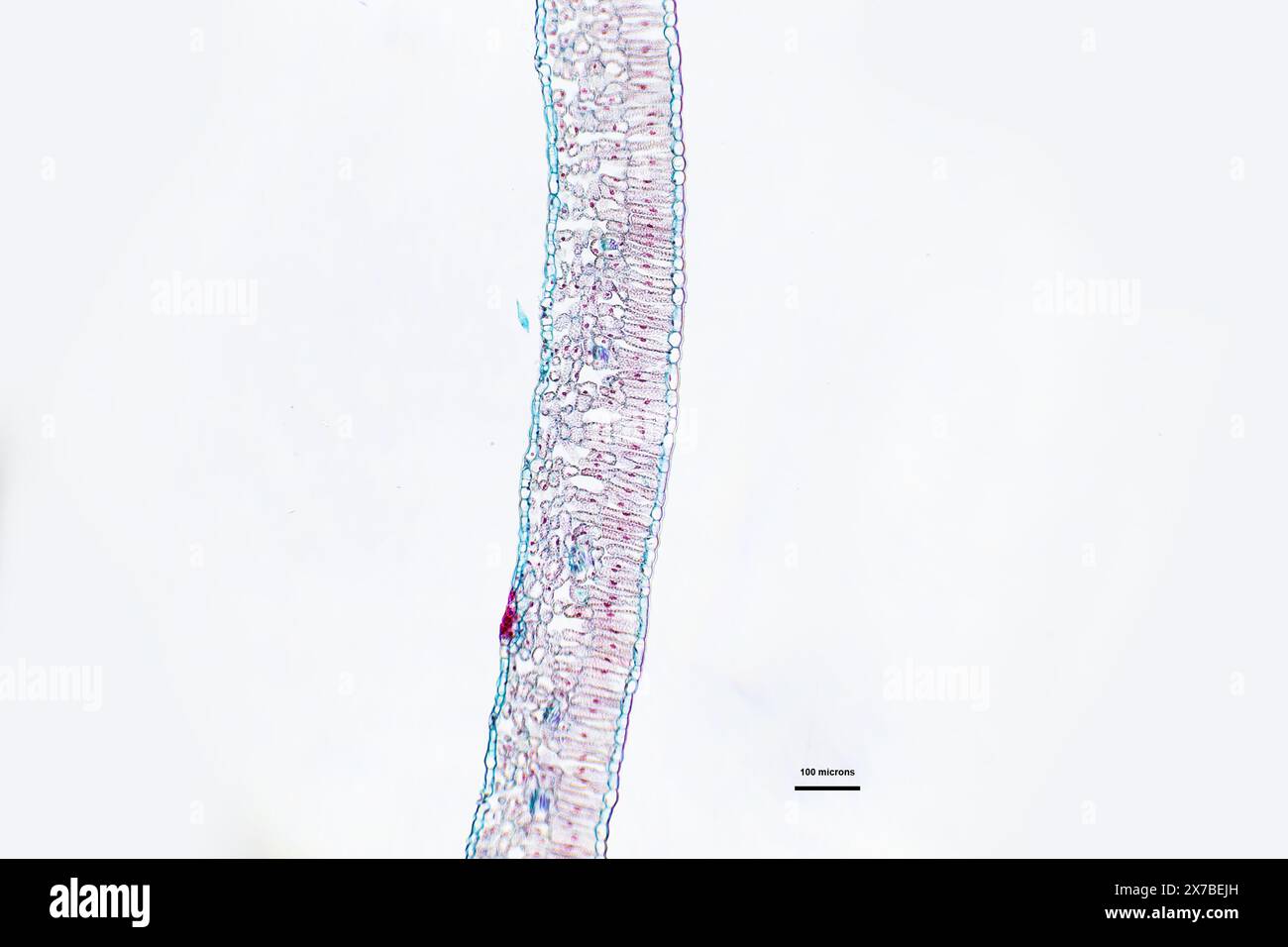 Privet leaf, transverse section under a microscope at 10 times magnification Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/privet-leaf-transverse-section-under-a-microscope-at-10-times-magnification-image606918441.html
Privet leaf, transverse section under a microscope at 10 times magnification Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/privet-leaf-transverse-section-under-a-microscope-at-10-times-magnification-image606918441.htmlRF2X7BEJH–Privet leaf, transverse section under a microscope at 10 times magnification
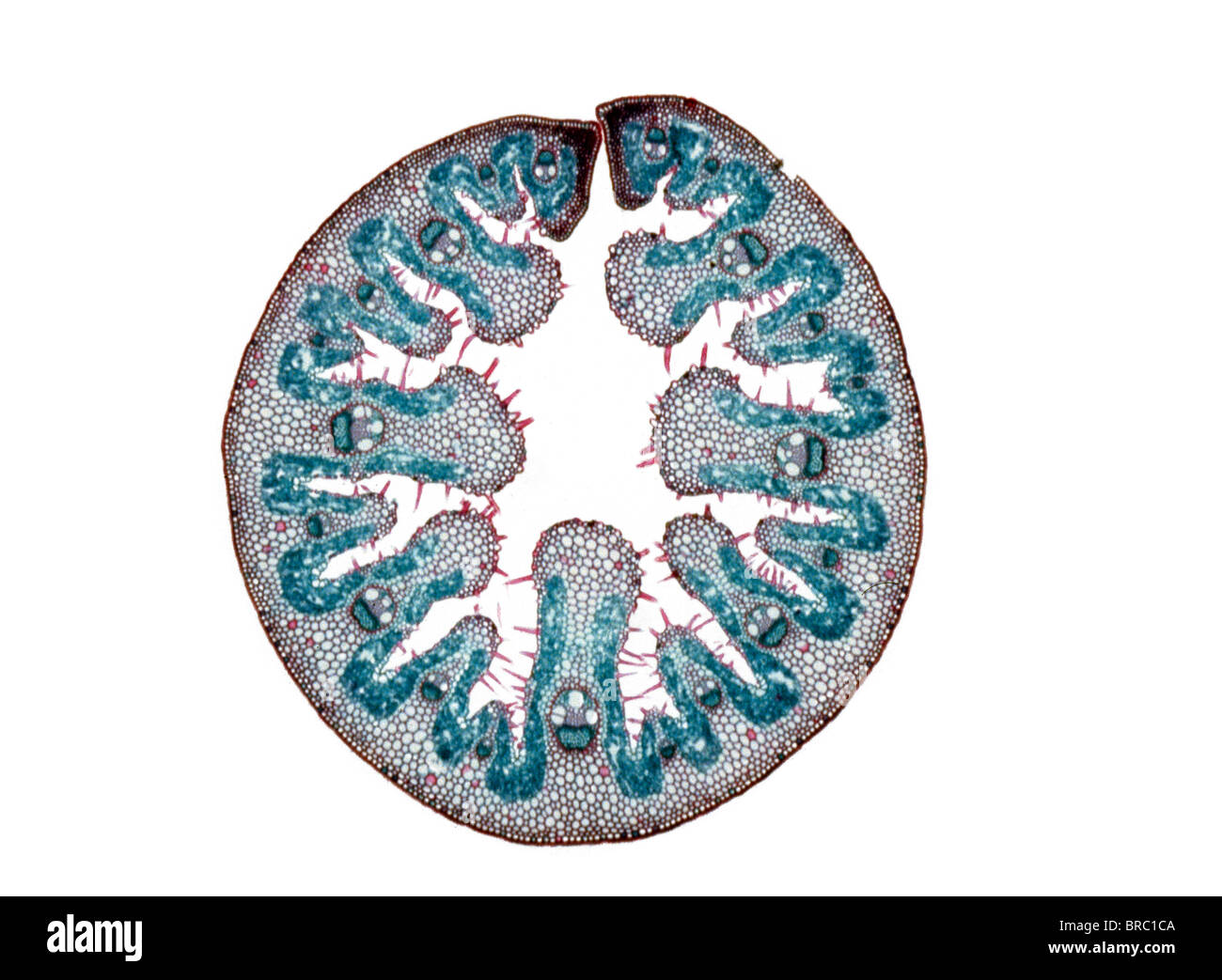 Light Micrograph (LM) of the transverse section of a leaf of Marram Grass (Ammophila sp.), magnification x 15 Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/stock-photo-light-micrograph-lm-of-the-transverse-section-of-a-leaf-of-marram-31612010.html
Light Micrograph (LM) of the transverse section of a leaf of Marram Grass (Ammophila sp.), magnification x 15 Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/stock-photo-light-micrograph-lm-of-the-transverse-section-of-a-leaf-of-marram-31612010.htmlRMBRC1CA–Light Micrograph (LM) of the transverse section of a leaf of Marram Grass (Ammophila sp.), magnification x 15
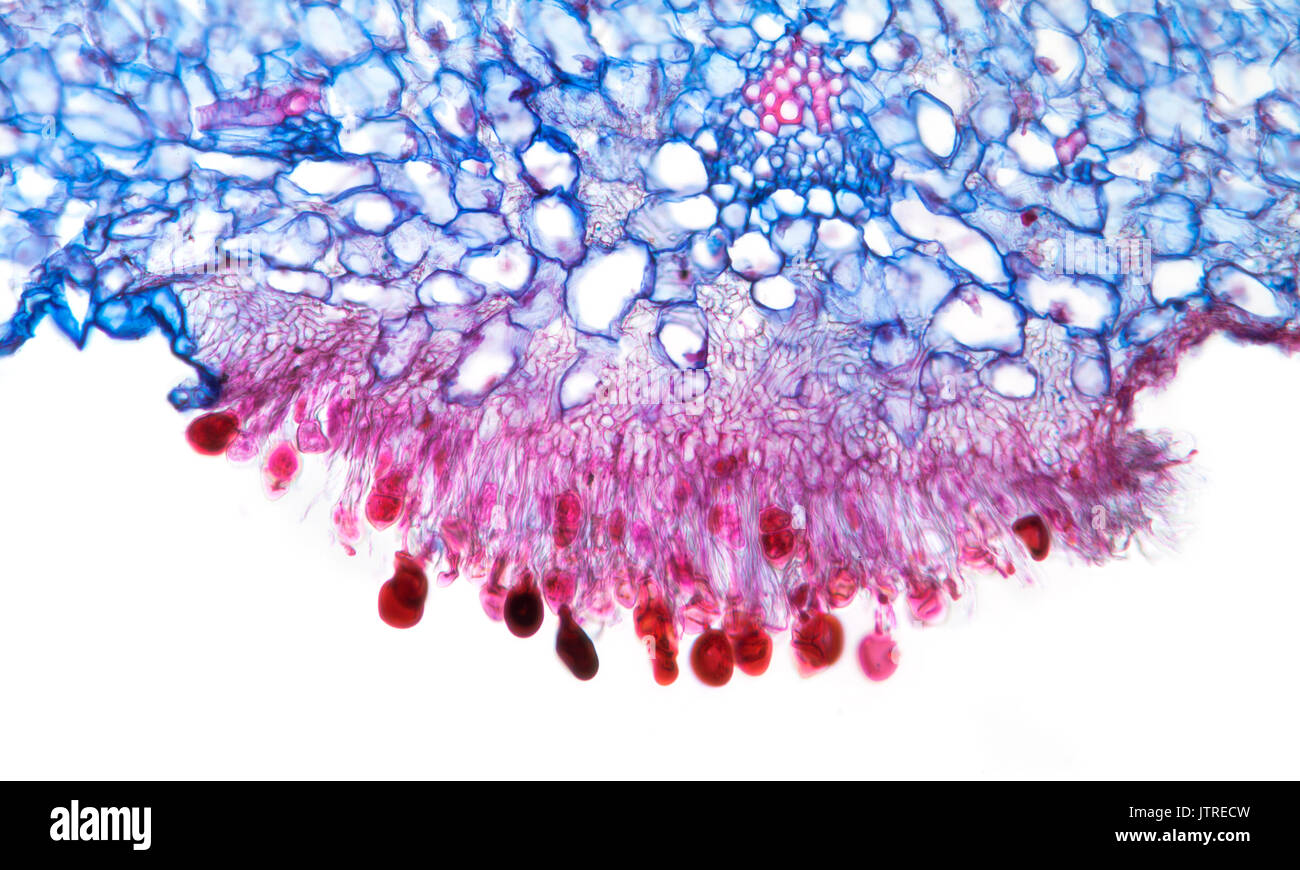 Snapdragon rust, Puccinia antirrhini fungus on antirrhinum leaf, brightfield photomicrograph, TS Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/snapdragon-rust-puccinia-antirrhini-fungus-on-antirrhinum-leaf-brightfield-image152950921.html
Snapdragon rust, Puccinia antirrhini fungus on antirrhinum leaf, brightfield photomicrograph, TS Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/snapdragon-rust-puccinia-antirrhini-fungus-on-antirrhinum-leaf-brightfield-image152950921.htmlRMJTRECW–Snapdragon rust, Puccinia antirrhini fungus on antirrhinum leaf, brightfield photomicrograph, TS
 biological cell isolated on whithe background microscope 3D Illustration Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/biological-cell-isolated-on-whithe-background-microscope-3d-illustration-image208953313.html
biological cell isolated on whithe background microscope 3D Illustration Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/biological-cell-isolated-on-whithe-background-microscope-3d-illustration-image208953313.htmlRFP3XJ29–biological cell isolated on whithe background microscope 3D Illustration
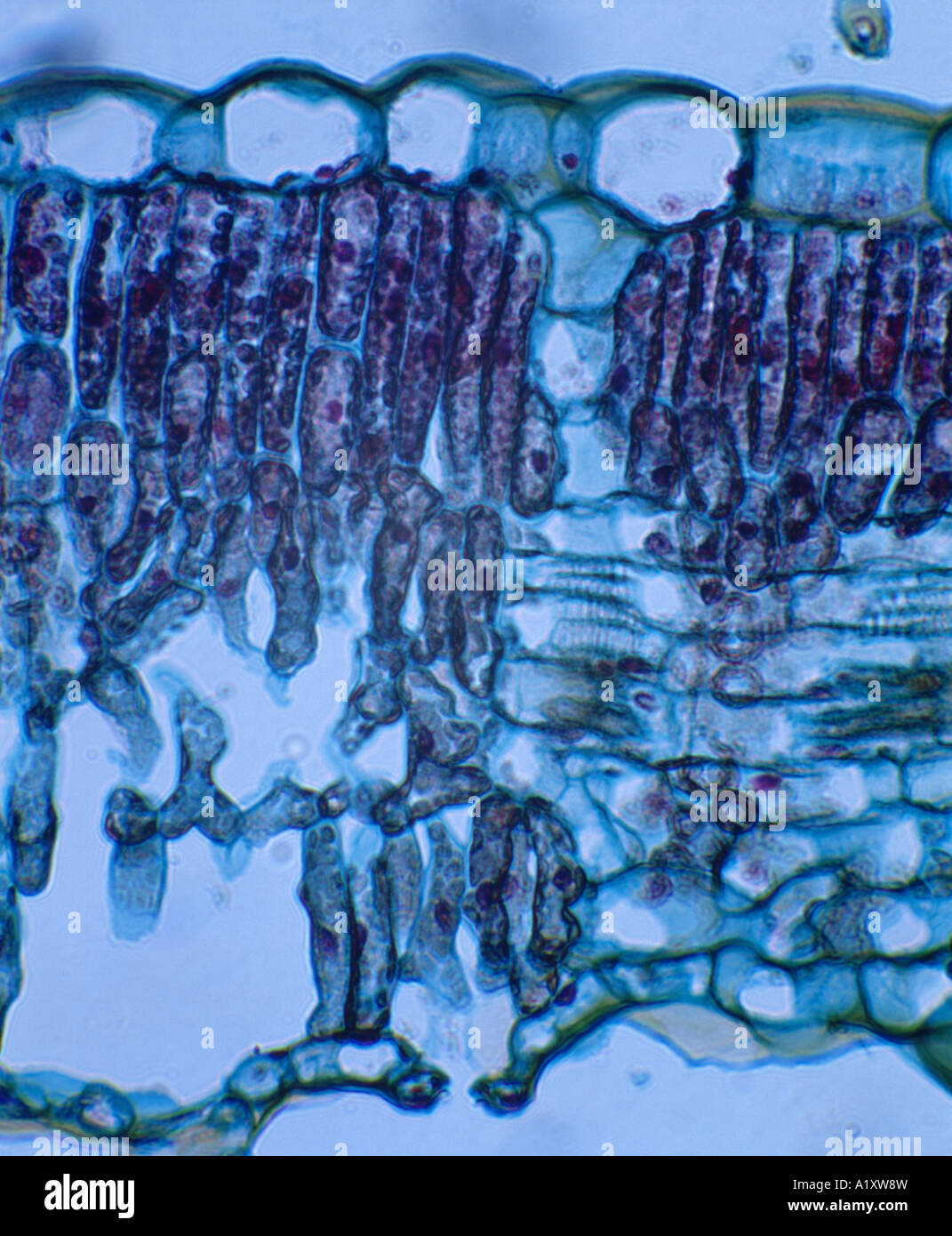 SUNFLOWER LEAF (HELIANTHUS SP.) CROSS SECTION SHOWING UPPER EPIDERMIS, PALASIDE CELLS, SPONGY LAYER, LOWER EPIDERMIS WITH GUARD Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/stock-photo-sunflower-leaf-helianthus-sp-cross-section-showing-upper-epidermis-10351032.html
SUNFLOWER LEAF (HELIANTHUS SP.) CROSS SECTION SHOWING UPPER EPIDERMIS, PALASIDE CELLS, SPONGY LAYER, LOWER EPIDERMIS WITH GUARD Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/stock-photo-sunflower-leaf-helianthus-sp-cross-section-showing-upper-epidermis-10351032.htmlRMA1XW8W–SUNFLOWER LEAF (HELIANTHUS SP.) CROSS SECTION SHOWING UPPER EPIDERMIS, PALASIDE CELLS, SPONGY LAYER, LOWER EPIDERMIS WITH GUARD
 Pine Leaf Mesophyll Cells Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/stock-photo-pine-leaf-mesophyll-cells-134943987.html
Pine Leaf Mesophyll Cells Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/stock-photo-pine-leaf-mesophyll-cells-134943987.htmlRMHRF6C3–Pine Leaf Mesophyll Cells
 Normal muscle, trichome Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/stock-photo-normal-muscle-trichome-73993868.html
Normal muscle, trichome Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/stock-photo-normal-muscle-trichome-73993868.htmlRFE8AKX4–Normal muscle, trichome
 Microscopy of a tree cell Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/microscopy-of-a-tree-cell-image454760432.html
Microscopy of a tree cell Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/microscopy-of-a-tree-cell-image454760432.htmlRF2HBT3G0–Microscopy of a tree cell
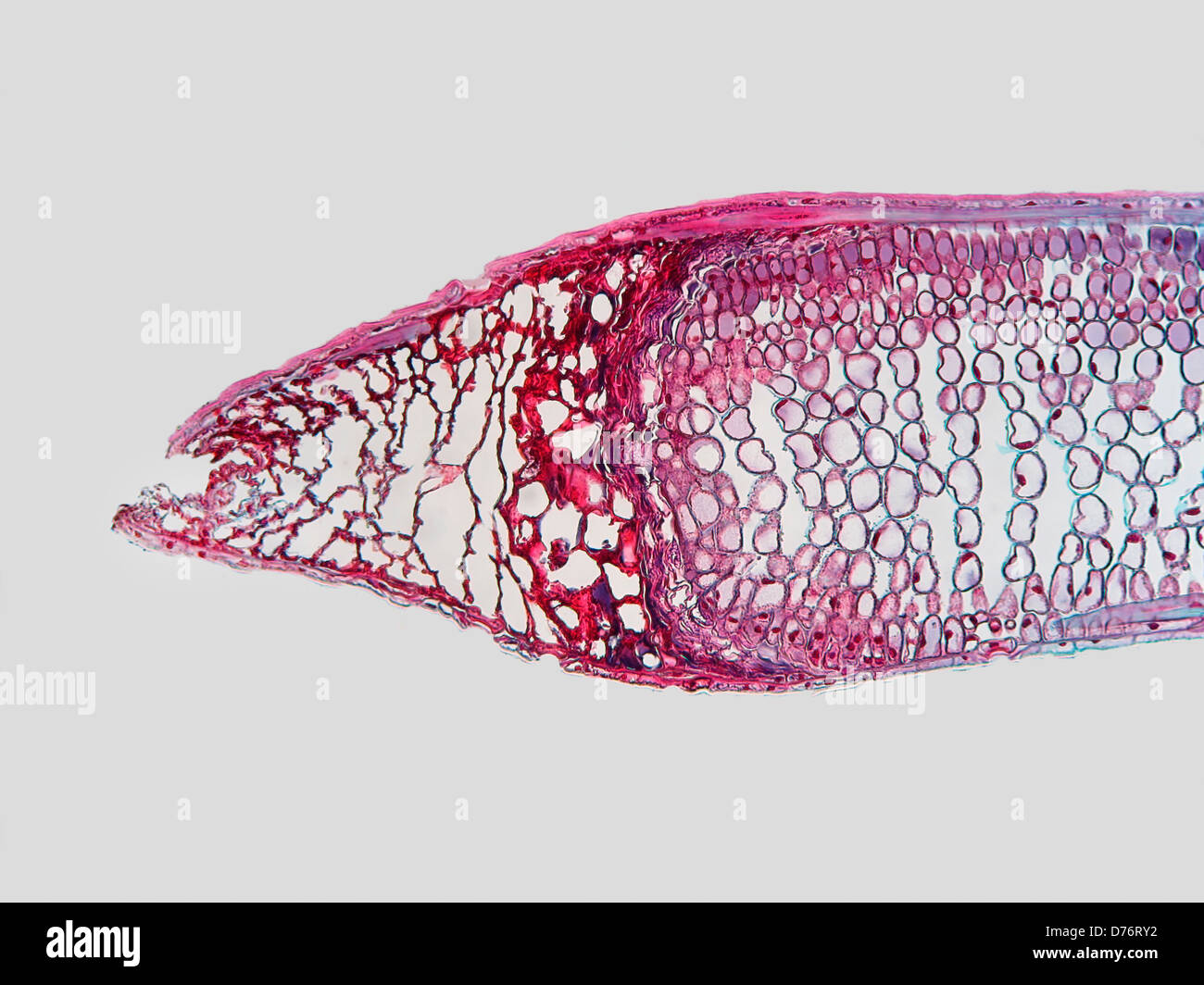 Longitudinal section fir needle that damaged before full growth attained magnification 100x Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/stock-photo-longitudinal-section-fir-needle-that-damaged-before-full-growth-attained-56084198.html
Longitudinal section fir needle that damaged before full growth attained magnification 100x Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/stock-photo-longitudinal-section-fir-needle-that-damaged-before-full-growth-attained-56084198.htmlRMD76RY2–Longitudinal section fir needle that damaged before full growth attained magnification 100x
 Box leaf. Coloured scanning electron micrograph (SEM) of a section through a leaf from the Common Box (Buxus sempervirens). The midrib (midvein) is the continuation of a leaf's stem along the centre of the leaf. At lower centre is a vascular bundle, which consists of xylem and phloem tissues. Xylem transports water and mineral nutrients from the roots throughout the plant and phloem transports carbohydrate and hormones around the plant. The surface (epidermis) of the leaf is covered in a waxy cuticle (top) that helps to prevent water loss. Magnification: x200 when printed 10 centimetres wide. Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/box-leaf-coloured-scanning-electron-micrograph-sem-of-a-section-through-a-leaf-from-the-common-box-buxus-sempervirens-the-midrib-midvein-is-the-continuation-of-a-leafs-stem-along-the-centre-of-the-leaf-at-lower-centre-is-a-vascular-bundle-which-consists-of-xylem-and-phloem-tissues-xylem-transports-water-and-mineral-nutrients-from-the-roots-throughout-the-plant-and-phloem-transports-carbohydrate-and-hormones-around-the-plant-the-surface-epidermis-of-the-leaf-is-covered-in-a-waxy-cuticle-top-that-helps-to-prevent-water-loss-magnification-x200-when-printed-10-centimetres-wide-image636416261.html
Box leaf. Coloured scanning electron micrograph (SEM) of a section through a leaf from the Common Box (Buxus sempervirens). The midrib (midvein) is the continuation of a leaf's stem along the centre of the leaf. At lower centre is a vascular bundle, which consists of xylem and phloem tissues. Xylem transports water and mineral nutrients from the roots throughout the plant and phloem transports carbohydrate and hormones around the plant. The surface (epidermis) of the leaf is covered in a waxy cuticle (top) that helps to prevent water loss. Magnification: x200 when printed 10 centimetres wide. Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/box-leaf-coloured-scanning-electron-micrograph-sem-of-a-section-through-a-leaf-from-the-common-box-buxus-sempervirens-the-midrib-midvein-is-the-continuation-of-a-leafs-stem-along-the-centre-of-the-leaf-at-lower-centre-is-a-vascular-bundle-which-consists-of-xylem-and-phloem-tissues-xylem-transports-water-and-mineral-nutrients-from-the-roots-throughout-the-plant-and-phloem-transports-carbohydrate-and-hormones-around-the-plant-the-surface-epidermis-of-the-leaf-is-covered-in-a-waxy-cuticle-top-that-helps-to-prevent-water-loss-magnification-x200-when-printed-10-centimetres-wide-image636416261.htmlRF2YYB7C5–Box leaf. Coloured scanning electron micrograph (SEM) of a section through a leaf from the Common Box (Buxus sempervirens). The midrib (midvein) is the continuation of a leaf's stem along the centre of the leaf. At lower centre is a vascular bundle, which consists of xylem and phloem tissues. Xylem transports water and mineral nutrients from the roots throughout the plant and phloem transports carbohydrate and hormones around the plant. The surface (epidermis) of the leaf is covered in a waxy cuticle (top) that helps to prevent water loss. Magnification: x200 when printed 10 centimetres wide.
 Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/-image225982129.html
Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/-image225982129.htmlRFR3JAE9–
 Tea leaf, light micrograph Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/stock-photo-tea-leaf-light-micrograph-33304993.html
Tea leaf, light micrograph Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/stock-photo-tea-leaf-light-micrograph-33304993.htmlRFBX54T1–Tea leaf, light micrograph
 Microscopy Photography. Flower of Capsella Bursa-pastoris. Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/microscopy-photography-flower-of-capsella-bursa-pastoris-image182996736.html
Microscopy Photography. Flower of Capsella Bursa-pastoris. Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/microscopy-photography-flower-of-capsella-bursa-pastoris-image182996736.htmlRFMHM65M–Microscopy Photography. Flower of Capsella Bursa-pastoris.
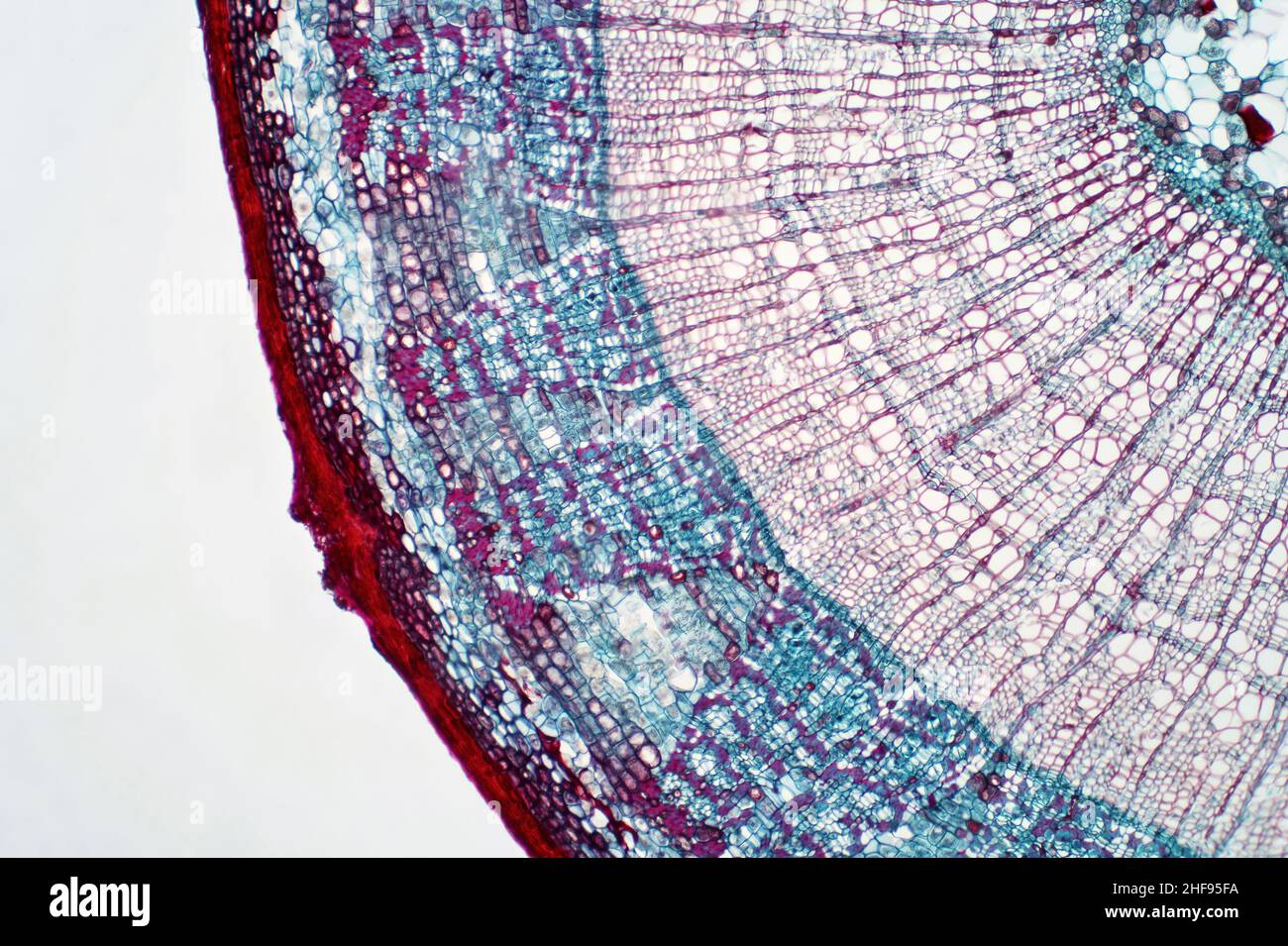 Plant stem, light micrograph Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/plant-stem-light-micrograph-image456891326.html
Plant stem, light micrograph Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/plant-stem-light-micrograph-image456891326.htmlRF2HF95FA–Plant stem, light micrograph
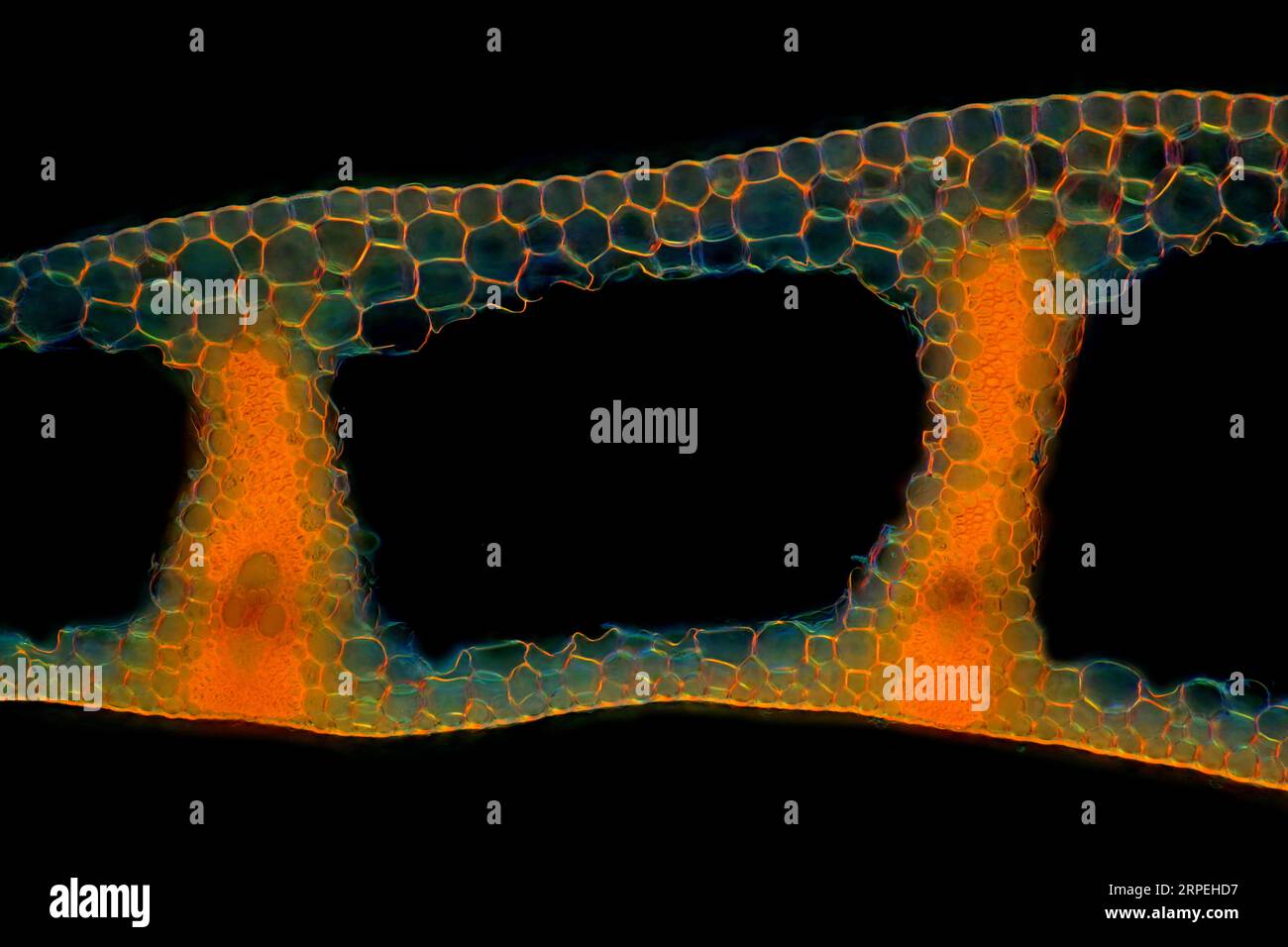 The image presents Carex sp. leaf in transversal cross-section, photographed through the microscope in polarized light at a magnification of 100X Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/the-image-presents-carex-sp-leaf-in-transversal-cross-section-photographed-through-the-microscope-in-polarized-light-at-a-magnification-of-100x-image564575235.html
The image presents Carex sp. leaf in transversal cross-section, photographed through the microscope in polarized light at a magnification of 100X Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/the-image-presents-carex-sp-leaf-in-transversal-cross-section-photographed-through-the-microscope-in-polarized-light-at-a-magnification-of-100x-image564575235.htmlRM2RPEHD7–The image presents Carex sp. leaf in transversal cross-section, photographed through the microscope in polarized light at a magnification of 100X
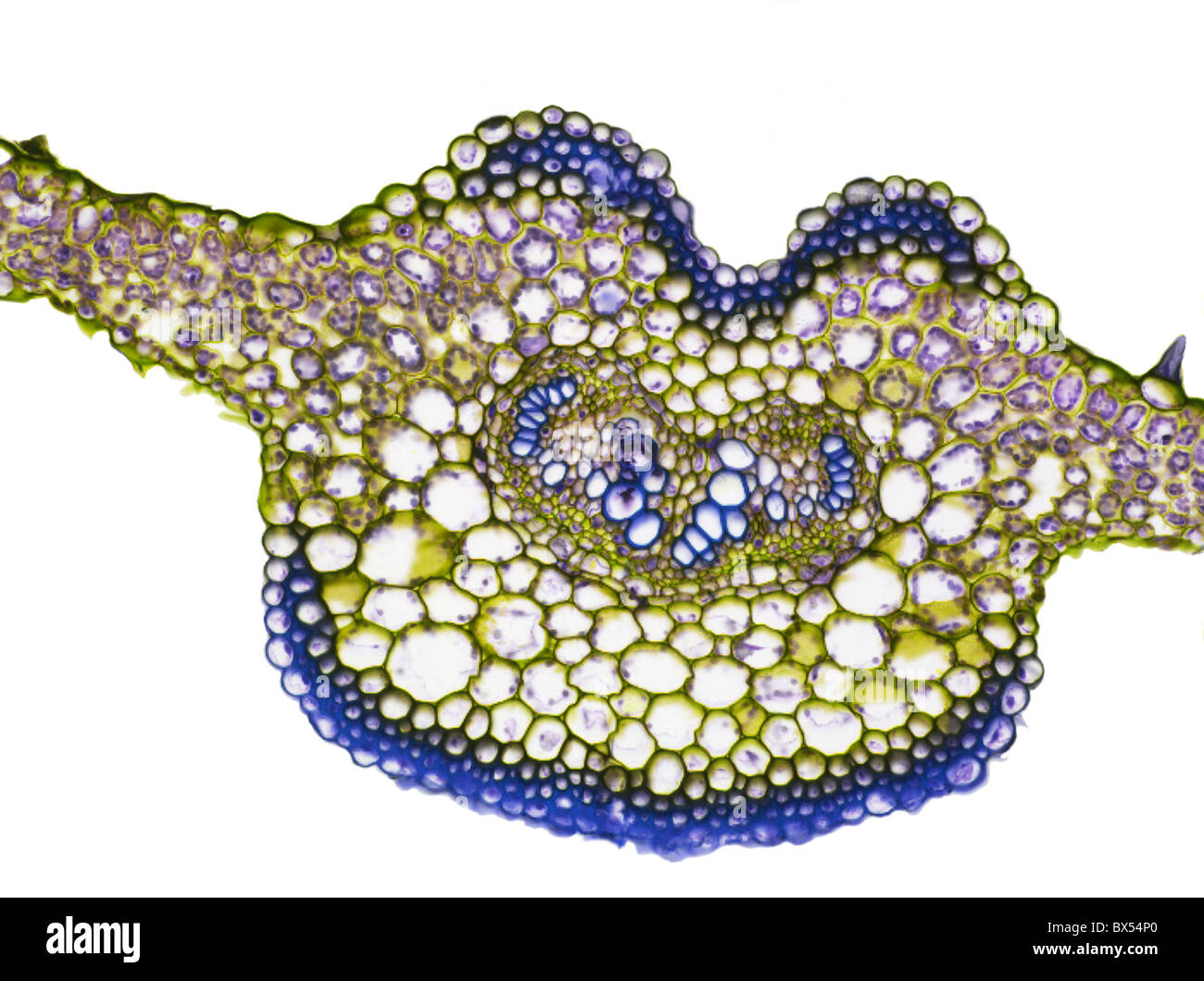 Fern leaf, light micrograph Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/stock-photo-fern-leaf-light-micrograph-33304936.html
Fern leaf, light micrograph Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/stock-photo-fern-leaf-light-micrograph-33304936.htmlRFBX54P0–Fern leaf, light micrograph
 Vicia Dicot leaf under a microscope (Vicia Dicot leaf W.M.), 40x Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/stock-photo-vicia-dicot-leaf-under-a-microscope-vicia-dicot-leaf-wm-40x-81101480.html
Vicia Dicot leaf under a microscope (Vicia Dicot leaf W.M.), 40x Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/stock-photo-vicia-dicot-leaf-under-a-microscope-vicia-dicot-leaf-wm-40x-81101480.htmlRFEKXDNC–Vicia Dicot leaf under a microscope (Vicia Dicot leaf W.M.), 40x
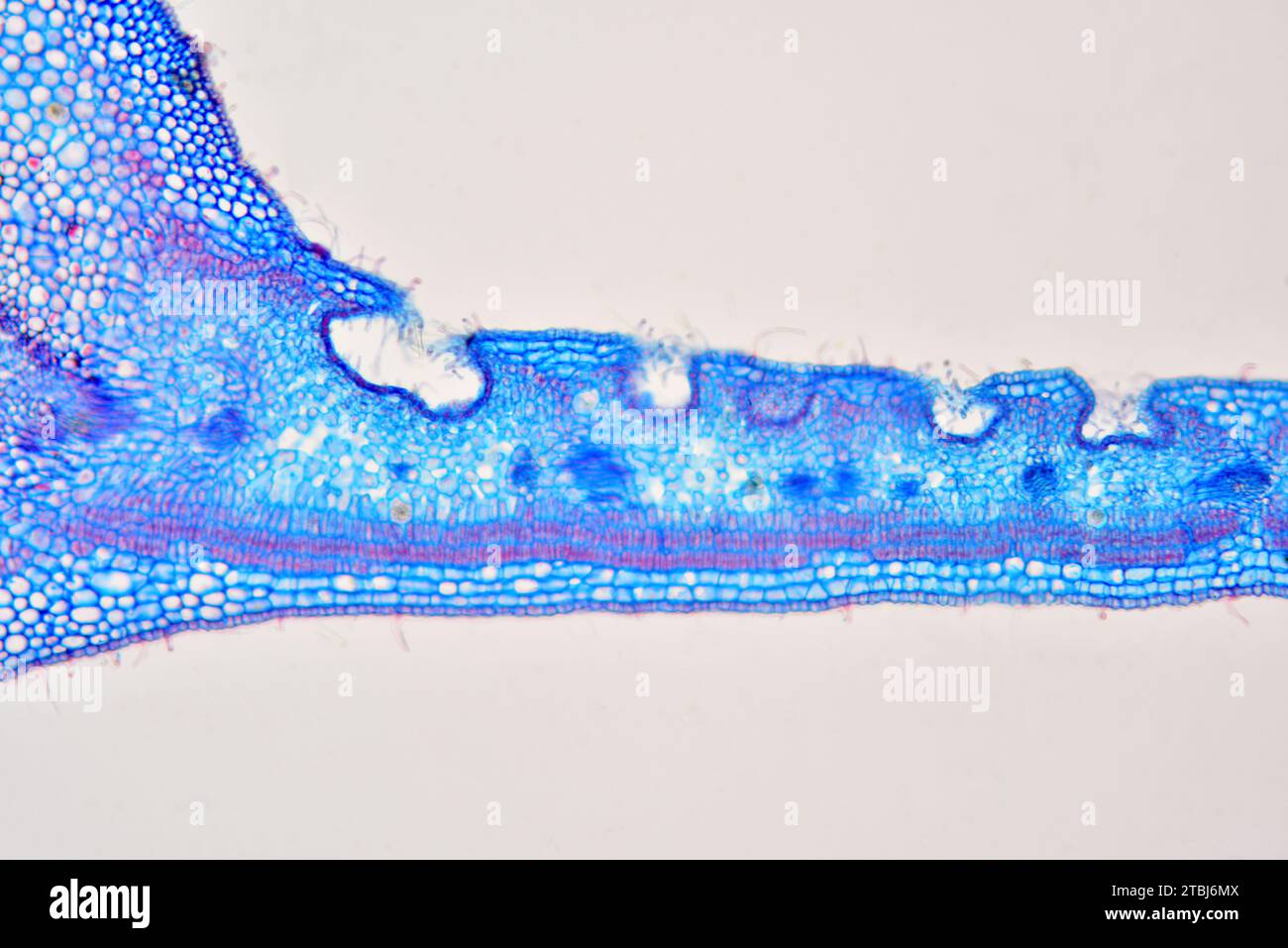 Leaf cross section of Nerium oleander showing epidermis, palisade mesophyll, spongy mesophyll and stomatal crypt. Optical microscope X100. Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/leaf-cross-section-of-nerium-oleander-showing-epidermis-palisade-mesophyll-spongy-mesophyll-and-stomatal-crypt-optical-microscope-x100-image575103786.html
Leaf cross section of Nerium oleander showing epidermis, palisade mesophyll, spongy mesophyll and stomatal crypt. Optical microscope X100. Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/leaf-cross-section-of-nerium-oleander-showing-epidermis-palisade-mesophyll-spongy-mesophyll-and-stomatal-crypt-optical-microscope-x100-image575103786.htmlRF2TBJ6MX–Leaf cross section of Nerium oleander showing epidermis, palisade mesophyll, spongy mesophyll and stomatal crypt. Optical microscope X100.
 science botany micrograph plant arabidopsis thaliana root tissue micro Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/stock-photo-science-botany-micrograph-plant-arabidopsis-thaliana-root-tissue-micro-50315324.html
science botany micrograph plant arabidopsis thaliana root tissue micro Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/stock-photo-science-botany-micrograph-plant-arabidopsis-thaliana-root-tissue-micro-50315324.htmlRFCWT1KT–science botany micrograph plant arabidopsis thaliana root tissue micro
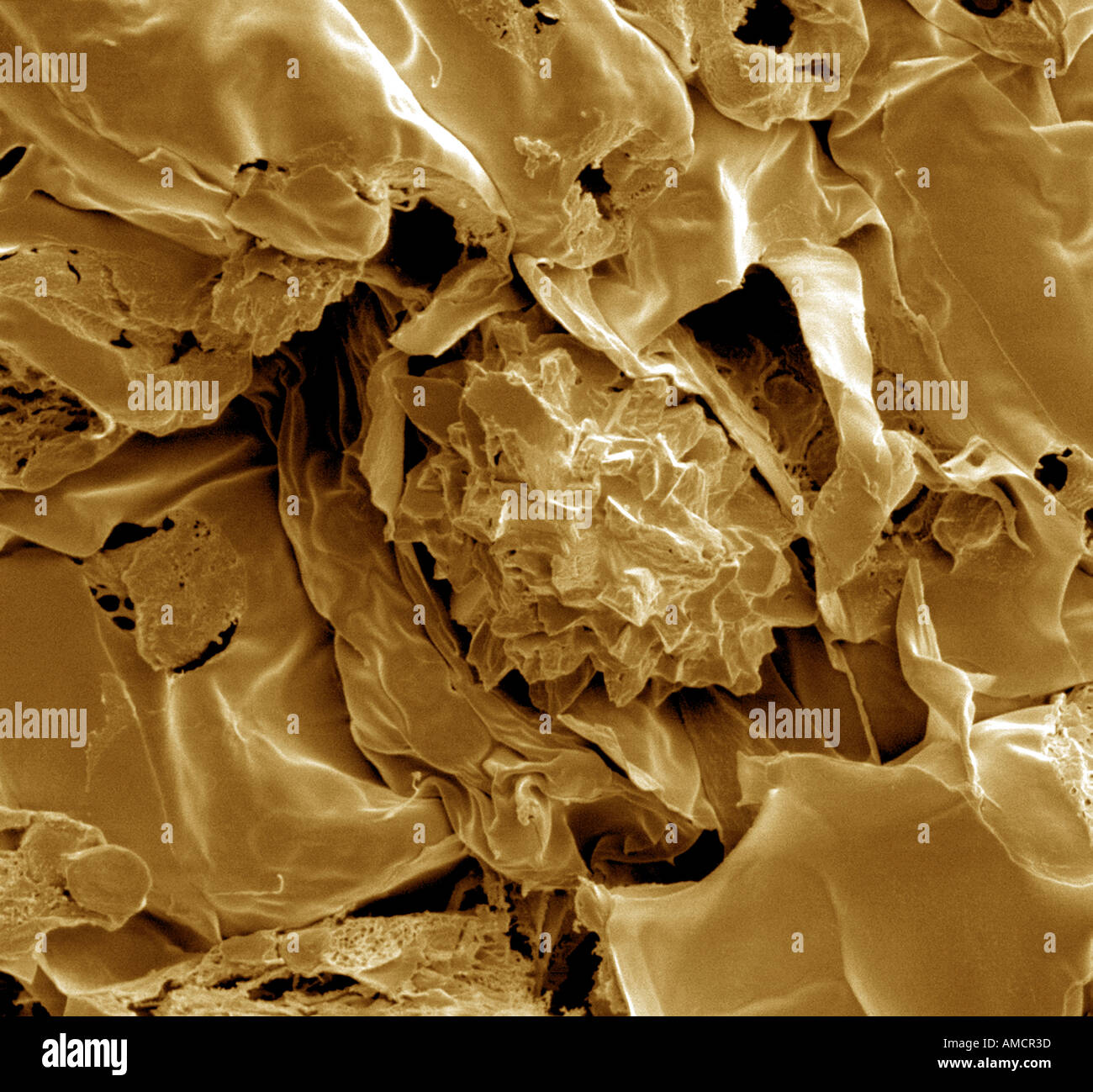 SEM x1000 - Dianthus leaf cross section Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/sem-x1000-dianthus-leaf-cross-section-image4970300.html
SEM x1000 - Dianthus leaf cross section Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/sem-x1000-dianthus-leaf-cross-section-image4970300.htmlRMAMCR3D–SEM x1000 - Dianthus leaf cross section
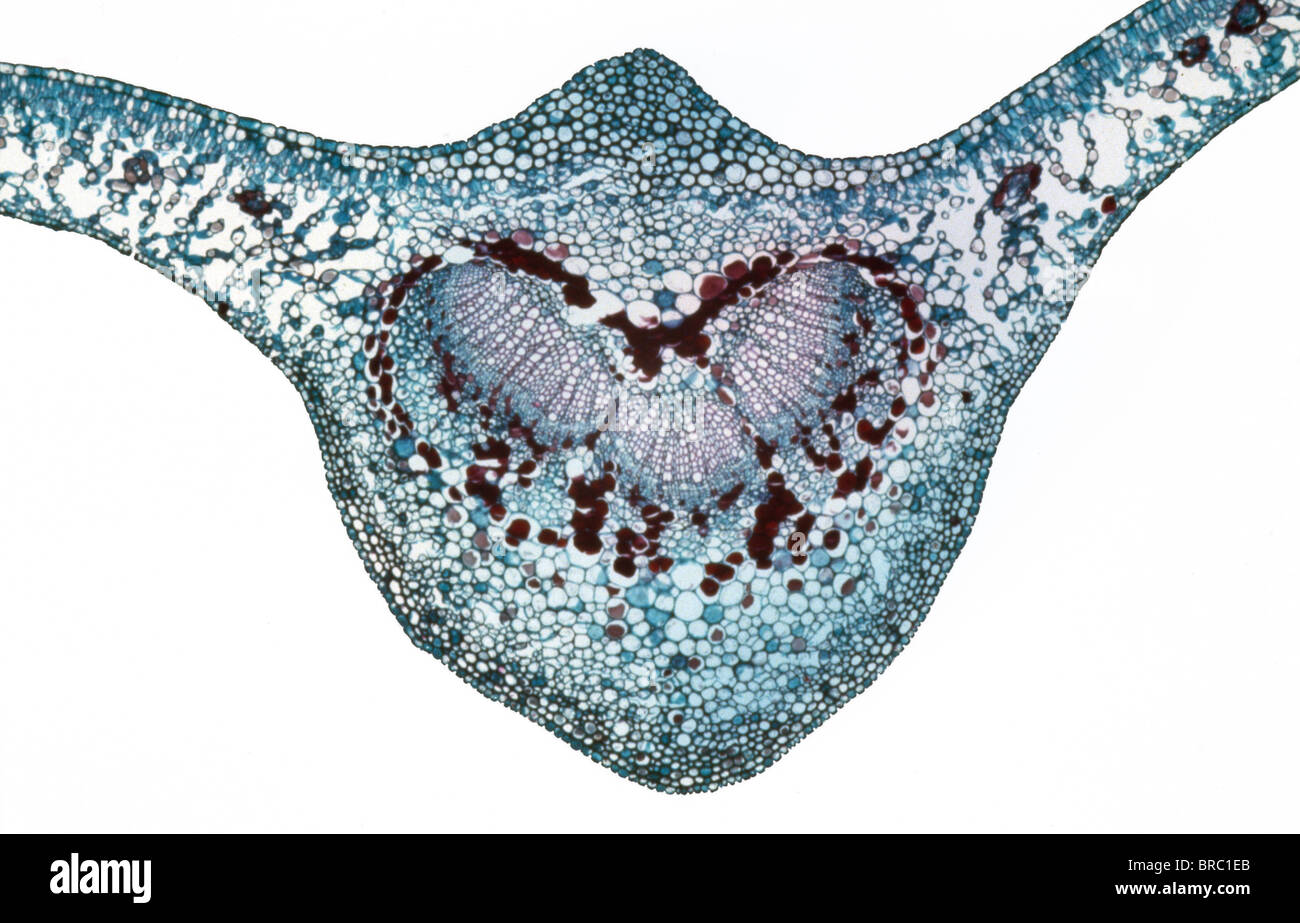 Light Micrograph (LM) of a transverse section of a leaf of a Cherry (Prunus sp.), magnification x 30 Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/stock-photo-light-micrograph-lm-of-a-transverse-section-of-a-leaf-of-a-cherry-31612067.html
Light Micrograph (LM) of a transverse section of a leaf of a Cherry (Prunus sp.), magnification x 30 Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/stock-photo-light-micrograph-lm-of-a-transverse-section-of-a-leaf-of-a-cherry-31612067.htmlRMBRC1EB–Light Micrograph (LM) of a transverse section of a leaf of a Cherry (Prunus sp.), magnification x 30
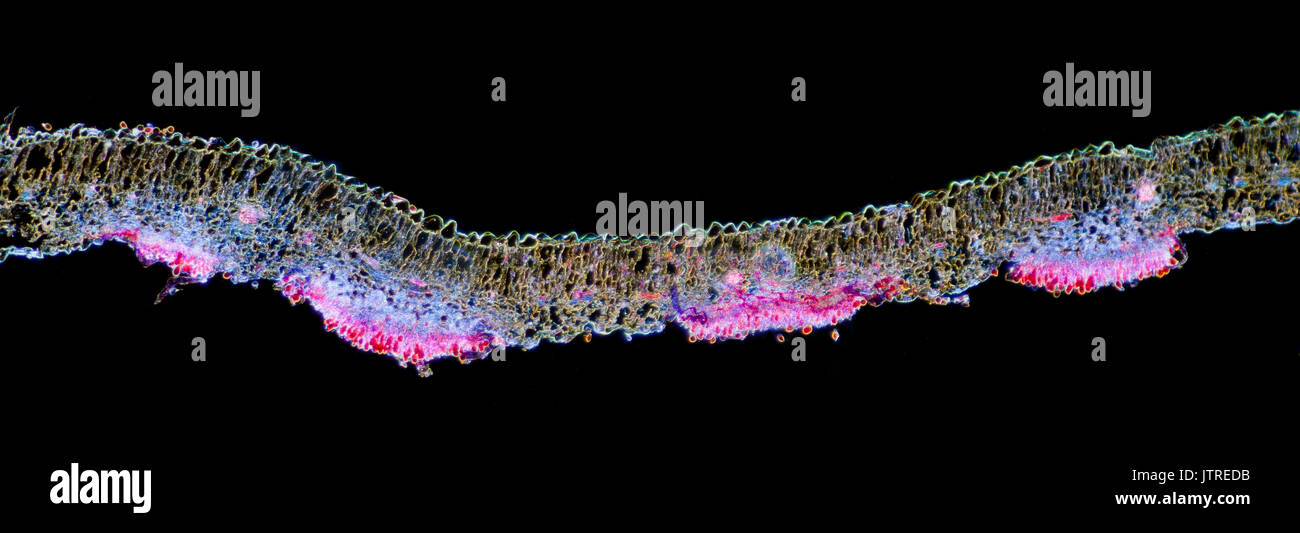 Snapdragon rust, Puccinia antirrhini fungus on antirrhinum leaf, darkfield photomicrograph, TS Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/snapdragon-rust-puccinia-antirrhini-fungus-on-antirrhinum-leaf-darkfield-image152950935.html
Snapdragon rust, Puccinia antirrhini fungus on antirrhinum leaf, darkfield photomicrograph, TS Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/snapdragon-rust-puccinia-antirrhini-fungus-on-antirrhinum-leaf-darkfield-image152950935.htmlRMJTREDB–Snapdragon rust, Puccinia antirrhini fungus on antirrhinum leaf, darkfield photomicrograph, TS
 biological cell isolated on whithe background microscope 3D Illustration Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/biological-cell-isolated-on-whithe-background-microscope-3d-illustration-image208953318.html
biological cell isolated on whithe background microscope 3D Illustration Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/biological-cell-isolated-on-whithe-background-microscope-3d-illustration-image208953318.htmlRFP3XJ2E–biological cell isolated on whithe background microscope 3D Illustration
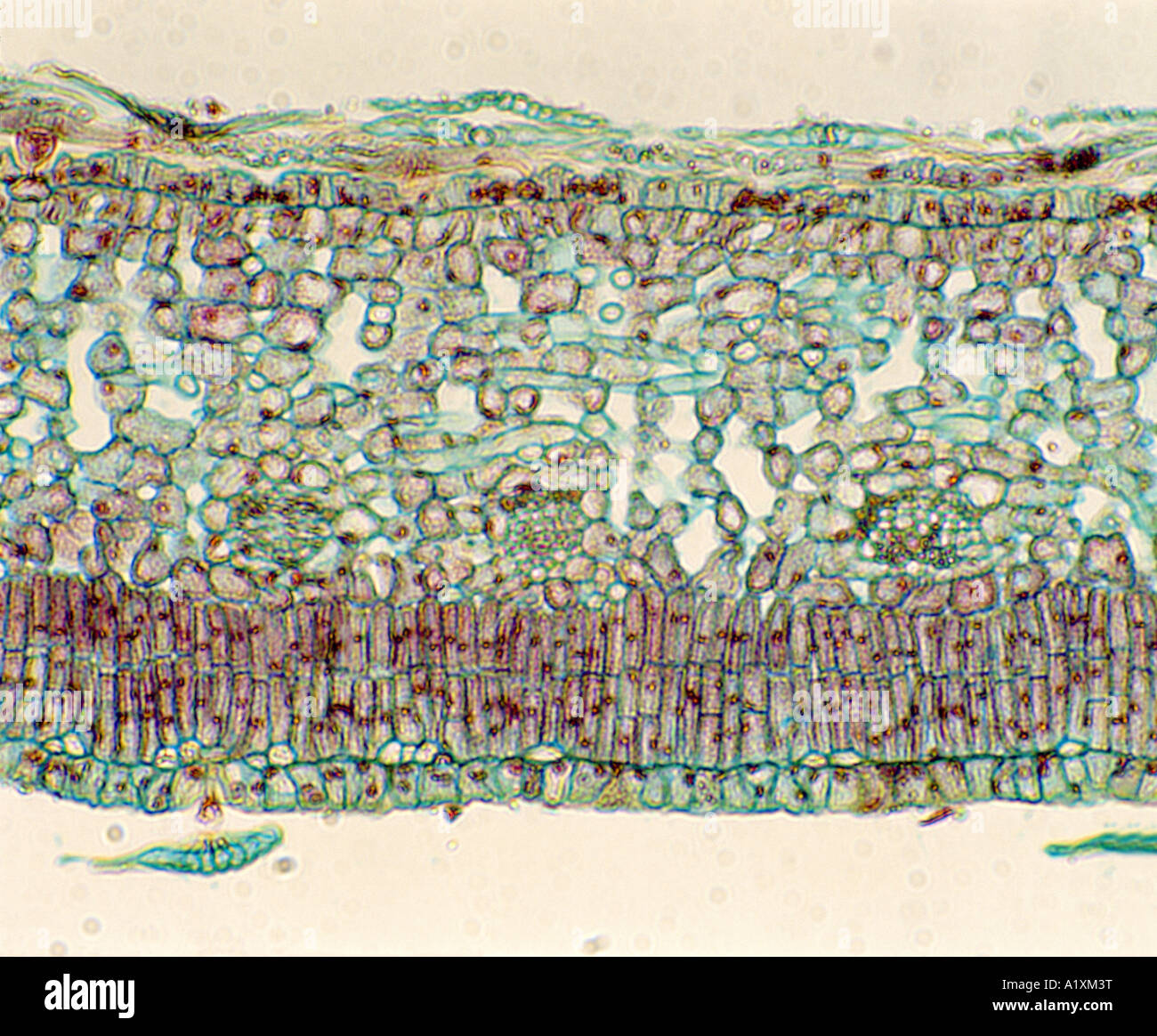 PUMPKIN (CUCURBITA SP.) LEAF CROSS SECTION; UPPER EPIDERMIS, PALISADE LAYER, SPONGY LAYER, VEINS (XYLEM & PHLOEM) LOWER EPIDERMI Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/stock-photo-pumpkin-cucurbita-sp-leaf-cross-section-upper-epidermis-palisade-layer-10349291.html
PUMPKIN (CUCURBITA SP.) LEAF CROSS SECTION; UPPER EPIDERMIS, PALISADE LAYER, SPONGY LAYER, VEINS (XYLEM & PHLOEM) LOWER EPIDERMI Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/stock-photo-pumpkin-cucurbita-sp-leaf-cross-section-upper-epidermis-palisade-layer-10349291.htmlRMA1XM3T–PUMPKIN (CUCURBITA SP.) LEAF CROSS SECTION; UPPER EPIDERMIS, PALISADE LAYER, SPONGY LAYER, VEINS (XYLEM & PHLOEM) LOWER EPIDERMI
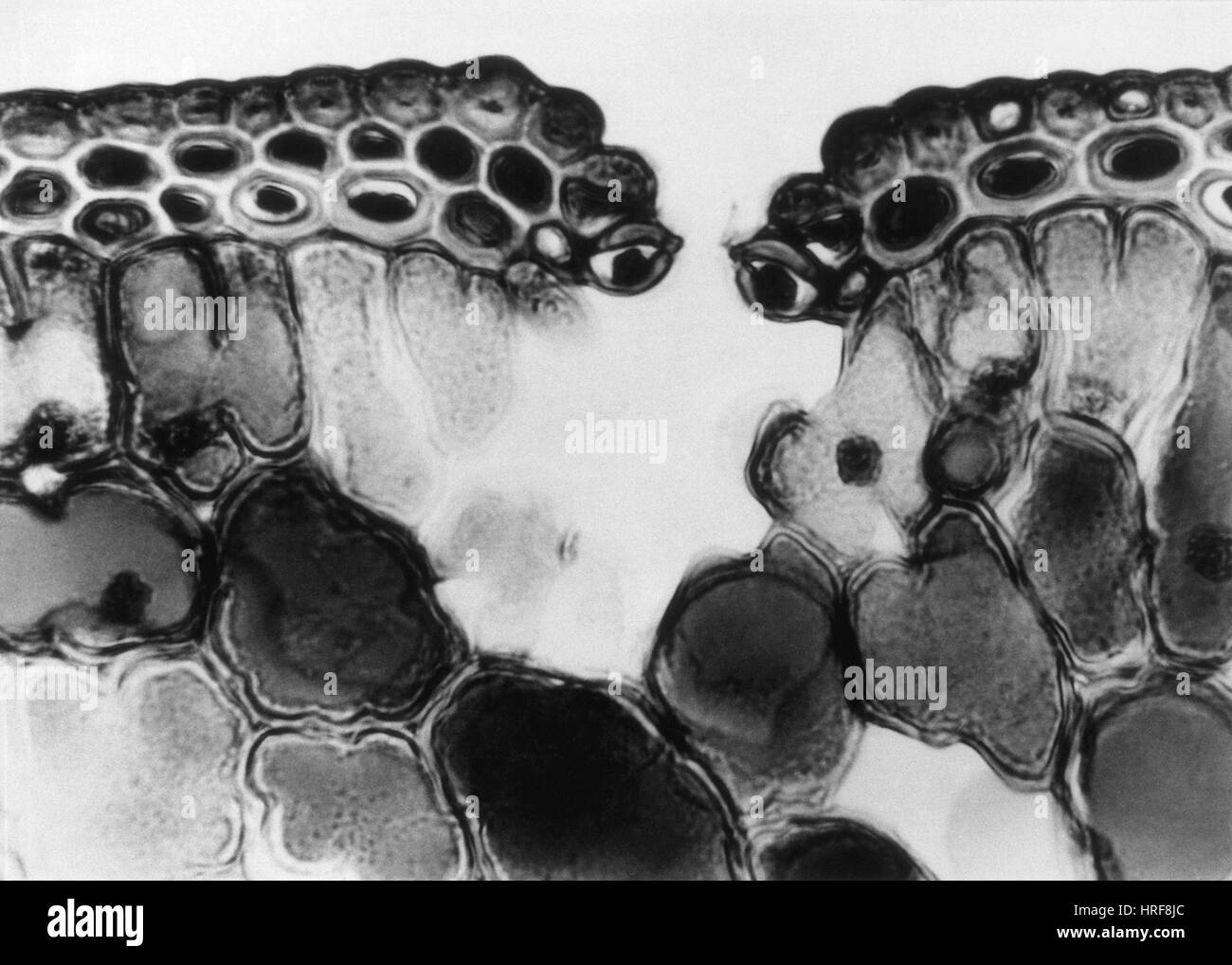 Cuticle and Epidermal Cells in Pine Leaf, LM Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/stock-photo-cuticle-and-epidermal-cells-in-pine-leaf-lm-134945732.html
Cuticle and Epidermal Cells in Pine Leaf, LM Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/stock-photo-cuticle-and-epidermal-cells-in-pine-leaf-lm-134945732.htmlRMHRF8JC–Cuticle and Epidermal Cells in Pine Leaf, LM
 Light photomicrograph of Leaf transversal section seen through microscope Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/stock-photo-light-photomicrograph-of-leaf-transversal-section-seen-through-microscope-75812195.html
Light photomicrograph of Leaf transversal section seen through microscope Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/stock-photo-light-photomicrograph-of-leaf-transversal-section-seen-through-microscope-75812195.htmlRFEB9F6B–Light photomicrograph of Leaf transversal section seen through microscope
 Archive image from page 61 of Cytological methods for the detection,. Cytological methods for the detection, identification, and characterization of orchid viruses and their inclusion bodies cytologicalmetho00kona Year: 1985 -55- Figure 55. Electron micrograph of virus particles distributed within the cytoplasm of CMV- infected Phalaenopsis leaf cells. Section was incubated with CMV antiserum (1/1000 dilution) and labelled with protein A-gold Pd:Plasmodesma. CW:Cell wall. Bar = 350 nm. Figure 56. Same tissue and treatments as in Fig. 55, except that incubation was with normal serum (1/500 Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/archive-image-from-page-61-of-cytological-methods-for-the-detection-cytological-methods-for-the-detection-identification-and-characterization-of-orchid-viruses-and-their-inclusion-bodies-cytologicalmetho00kona-year-1985-55-figure-55-electron-micrograph-of-virus-particles-distributed-within-the-cytoplasm-of-cmv-infected-phalaenopsis-leaf-cells-section-was-incubated-with-cmv-antiserum-11000-dilution-and-labelled-with-protein-a-gold-pdplasmodesma-cwcell-wall-bar-=-350-nm-figure-56-same-tissue-and-treatments-as-in-fig-55-except-that-incubation-was-with-normal-serum-1500-image259288015.html
Archive image from page 61 of Cytological methods for the detection,. Cytological methods for the detection, identification, and characterization of orchid viruses and their inclusion bodies cytologicalmetho00kona Year: 1985 -55- Figure 55. Electron micrograph of virus particles distributed within the cytoplasm of CMV- infected Phalaenopsis leaf cells. Section was incubated with CMV antiserum (1/1000 dilution) and labelled with protein A-gold Pd:Plasmodesma. CW:Cell wall. Bar = 350 nm. Figure 56. Same tissue and treatments as in Fig. 55, except that incubation was with normal serum (1/500 Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/archive-image-from-page-61-of-cytological-methods-for-the-detection-cytological-methods-for-the-detection-identification-and-characterization-of-orchid-viruses-and-their-inclusion-bodies-cytologicalmetho00kona-year-1985-55-figure-55-electron-micrograph-of-virus-particles-distributed-within-the-cytoplasm-of-cmv-infected-phalaenopsis-leaf-cells-section-was-incubated-with-cmv-antiserum-11000-dilution-and-labelled-with-protein-a-gold-pdplasmodesma-cwcell-wall-bar-=-350-nm-figure-56-same-tissue-and-treatments-as-in-fig-55-except-that-incubation-was-with-normal-serum-1500-image259288015.htmlRMW1RGE7–Archive image from page 61 of Cytological methods for the detection,. Cytological methods for the detection, identification, and characterization of orchid viruses and their inclusion bodies cytologicalmetho00kona Year: 1985 -55- Figure 55. Electron micrograph of virus particles distributed within the cytoplasm of CMV- infected Phalaenopsis leaf cells. Section was incubated with CMV antiserum (1/1000 dilution) and labelled with protein A-gold Pd:Plasmodesma. CW:Cell wall. Bar = 350 nm. Figure 56. Same tissue and treatments as in Fig. 55, except that incubation was with normal serum (1/500
 Portion longitudinal section pine needle showing stomata on both upper lower surfaces magnification 200x Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/stock-photo-portion-longitudinal-section-pine-needle-showing-stomata-on-both-upper-56084297.html
Portion longitudinal section pine needle showing stomata on both upper lower surfaces magnification 200x Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/stock-photo-portion-longitudinal-section-pine-needle-showing-stomata-on-both-upper-56084297.htmlRMD76T2H–Portion longitudinal section pine needle showing stomata on both upper lower surfaces magnification 200x
 . Cytological methods for the detection, identification, and characterization of orchid viruses and their inclusion bodies. Orchids; Virus diseases of plants. -53- « * sr >. Figure 51. Electron micrograph of virus aggregations in CyMV-infected Cymbidium leaf cell showing the presence of gold particles on their surface. Section is incubated with CyMV antiserum (1/2000 dilution) and labelled with protein A-gold. Bar = 260 nm, Figure 52. Electron micrograph of virus-like structures in the same antiserum-treated section as in Fig. 51, but showing no specific reaction. Same treatment as in Fig. Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/cytological-methods-for-the-detection-identification-and-characterization-of-orchid-viruses-and-their-inclusion-bodies-orchids-virus-diseases-of-plants-53-sr-gt-figure-51-electron-micrograph-of-virus-aggregations-in-cymv-infected-cymbidium-leaf-cell-showing-the-presence-of-gold-particles-on-their-surface-section-is-incubated-with-cymv-antiserum-12000-dilution-and-labelled-with-protein-a-gold-bar-=-260-nm-figure-52-electron-micrograph-of-virus-like-structures-in-the-same-antiserum-treated-section-as-in-fig-51-but-showing-no-specific-reaction-same-treatment-as-in-fig-image216162201.html
. Cytological methods for the detection, identification, and characterization of orchid viruses and their inclusion bodies. Orchids; Virus diseases of plants. -53- « * sr >. Figure 51. Electron micrograph of virus aggregations in CyMV-infected Cymbidium leaf cell showing the presence of gold particles on their surface. Section is incubated with CyMV antiserum (1/2000 dilution) and labelled with protein A-gold. Bar = 260 nm, Figure 52. Electron micrograph of virus-like structures in the same antiserum-treated section as in Fig. 51, but showing no specific reaction. Same treatment as in Fig. Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/cytological-methods-for-the-detection-identification-and-characterization-of-orchid-viruses-and-their-inclusion-bodies-orchids-virus-diseases-of-plants-53-sr-gt-figure-51-electron-micrograph-of-virus-aggregations-in-cymv-infected-cymbidium-leaf-cell-showing-the-presence-of-gold-particles-on-their-surface-section-is-incubated-with-cymv-antiserum-12000-dilution-and-labelled-with-protein-a-gold-bar-=-260-nm-figure-52-electron-micrograph-of-virus-like-structures-in-the-same-antiserum-treated-section-as-in-fig-51-but-showing-no-specific-reaction-same-treatment-as-in-fig-image216162201.htmlRMPFK12H–. Cytological methods for the detection, identification, and characterization of orchid viruses and their inclusion bodies. Orchids; Virus diseases of plants. -53- « * sr >. Figure 51. Electron micrograph of virus aggregations in CyMV-infected Cymbidium leaf cell showing the presence of gold particles on their surface. Section is incubated with CyMV antiserum (1/2000 dilution) and labelled with protein A-gold. Bar = 260 nm, Figure 52. Electron micrograph of virus-like structures in the same antiserum-treated section as in Fig. 51, but showing no specific reaction. Same treatment as in Fig.
 Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/-image225982130.html
Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/-image225982130.htmlRFR3JAEA–
 . Cytological methods for the detection, identification, and characterization of orchid viruses and their inclusion bodies. Orchids; Virus diseases of plants. -55-. Figure 55. Electron micrograph of virus particles distributed within the cytoplasm of CMV- infected Phalaenopsis leaf cells. Section was incubated with CMV antiserum (1/1000 dilution) and labelled with protein A-gold Pd:Plasmodesma. CW:Cell wall. Bar = 350 nm. Figure 56. Same tissue and treatments as in Fig. 55, except that incubation was with normal serum (1/500 dilution). Note the background of nonspecific reaction (arrows). CWrC Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/cytological-methods-for-the-detection-identification-and-characterization-of-orchid-viruses-and-their-inclusion-bodies-orchids-virus-diseases-of-plants-55-figure-55-electron-micrograph-of-virus-particles-distributed-within-the-cytoplasm-of-cmv-infected-phalaenopsis-leaf-cells-section-was-incubated-with-cmv-antiserum-11000-dilution-and-labelled-with-protein-a-gold-pdplasmodesma-cwcell-wall-bar-=-350-nm-figure-56-same-tissue-and-treatments-as-in-fig-55-except-that-incubation-was-with-normal-serum-1500-dilution-note-the-background-of-nonspecific-reaction-arrows-cwrc-image231779156.html
. Cytological methods for the detection, identification, and characterization of orchid viruses and their inclusion bodies. Orchids; Virus diseases of plants. -55-. Figure 55. Electron micrograph of virus particles distributed within the cytoplasm of CMV- infected Phalaenopsis leaf cells. Section was incubated with CMV antiserum (1/1000 dilution) and labelled with protein A-gold Pd:Plasmodesma. CW:Cell wall. Bar = 350 nm. Figure 56. Same tissue and treatments as in Fig. 55, except that incubation was with normal serum (1/500 dilution). Note the background of nonspecific reaction (arrows). CWrC Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/cytological-methods-for-the-detection-identification-and-characterization-of-orchid-viruses-and-their-inclusion-bodies-orchids-virus-diseases-of-plants-55-figure-55-electron-micrograph-of-virus-particles-distributed-within-the-cytoplasm-of-cmv-infected-phalaenopsis-leaf-cells-section-was-incubated-with-cmv-antiserum-11000-dilution-and-labelled-with-protein-a-gold-pdplasmodesma-cwcell-wall-bar-=-350-nm-figure-56-same-tissue-and-treatments-as-in-fig-55-except-that-incubation-was-with-normal-serum-1500-dilution-note-the-background-of-nonspecific-reaction-arrows-cwrc-image231779156.htmlRMRD2CK0–. Cytological methods for the detection, identification, and characterization of orchid viruses and their inclusion bodies. Orchids; Virus diseases of plants. -55-. Figure 55. Electron micrograph of virus particles distributed within the cytoplasm of CMV- infected Phalaenopsis leaf cells. Section was incubated with CMV antiserum (1/1000 dilution) and labelled with protein A-gold Pd:Plasmodesma. CW:Cell wall. Bar = 350 nm. Figure 56. Same tissue and treatments as in Fig. 55, except that incubation was with normal serum (1/500 dilution). Note the background of nonspecific reaction (arrows). CWrC
 Fern leaf, light micrograph Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/stock-photo-fern-leaf-light-micrograph-33304934.html
Fern leaf, light micrograph Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/stock-photo-fern-leaf-light-micrograph-33304934.htmlRFBX54NX–Fern leaf, light micrograph
 Vicia Dicot leaf under a microscope (Vicia Dicot leaf W.M.), 40x Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/stock-photo-vicia-dicot-leaf-under-a-microscope-vicia-dicot-leaf-wm-40x-80958638.html
Vicia Dicot leaf under a microscope (Vicia Dicot leaf W.M.), 40x Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/stock-photo-vicia-dicot-leaf-under-a-microscope-vicia-dicot-leaf-wm-40x-80958638.htmlRFEKKYFX–Vicia Dicot leaf under a microscope (Vicia Dicot leaf W.M.), 40x
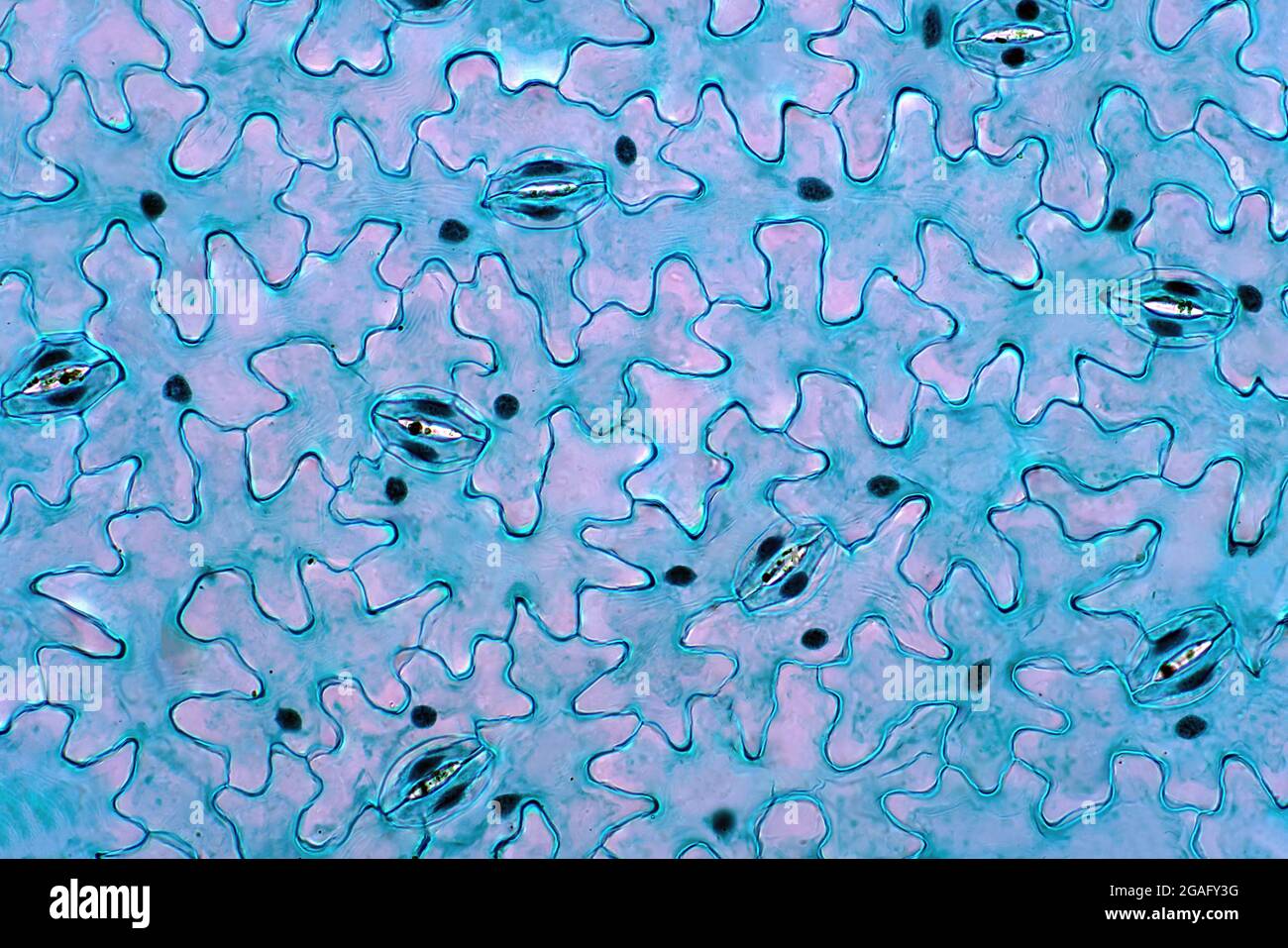 Leaf epidermis, light micrograph Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/leaf-epidermis-light-micrograph-image436756308.html
Leaf epidermis, light micrograph Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/leaf-epidermis-light-micrograph-image436756308.htmlRF2GAFY3G–Leaf epidermis, light micrograph
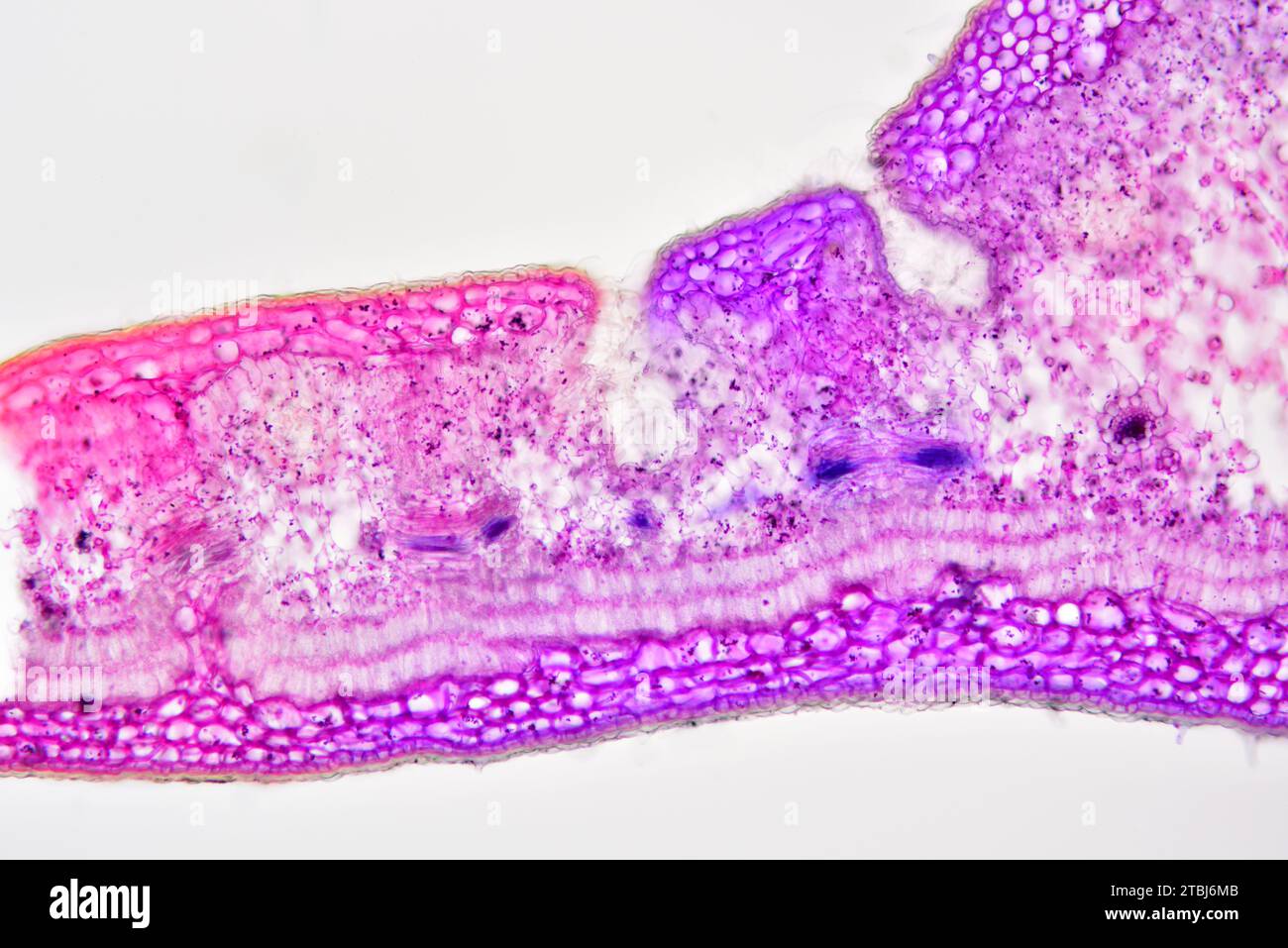 Leaf cross section of Nerium oleander showing epidermis, palisade mesophyll, spongy mesophyll, vascular bundle, phloem, xylem, collenchyma and stomata Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/leaf-cross-section-of-nerium-oleander-showing-epidermis-palisade-mesophyll-spongy-mesophyll-vascular-bundle-phloem-xylem-collenchyma-and-stomata-image575103771.html
Leaf cross section of Nerium oleander showing epidermis, palisade mesophyll, spongy mesophyll, vascular bundle, phloem, xylem, collenchyma and stomata Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/leaf-cross-section-of-nerium-oleander-showing-epidermis-palisade-mesophyll-spongy-mesophyll-vascular-bundle-phloem-xylem-collenchyma-and-stomata-image575103771.htmlRF2TBJ6MB–Leaf cross section of Nerium oleander showing epidermis, palisade mesophyll, spongy mesophyll, vascular bundle, phloem, xylem, collenchyma and stomata
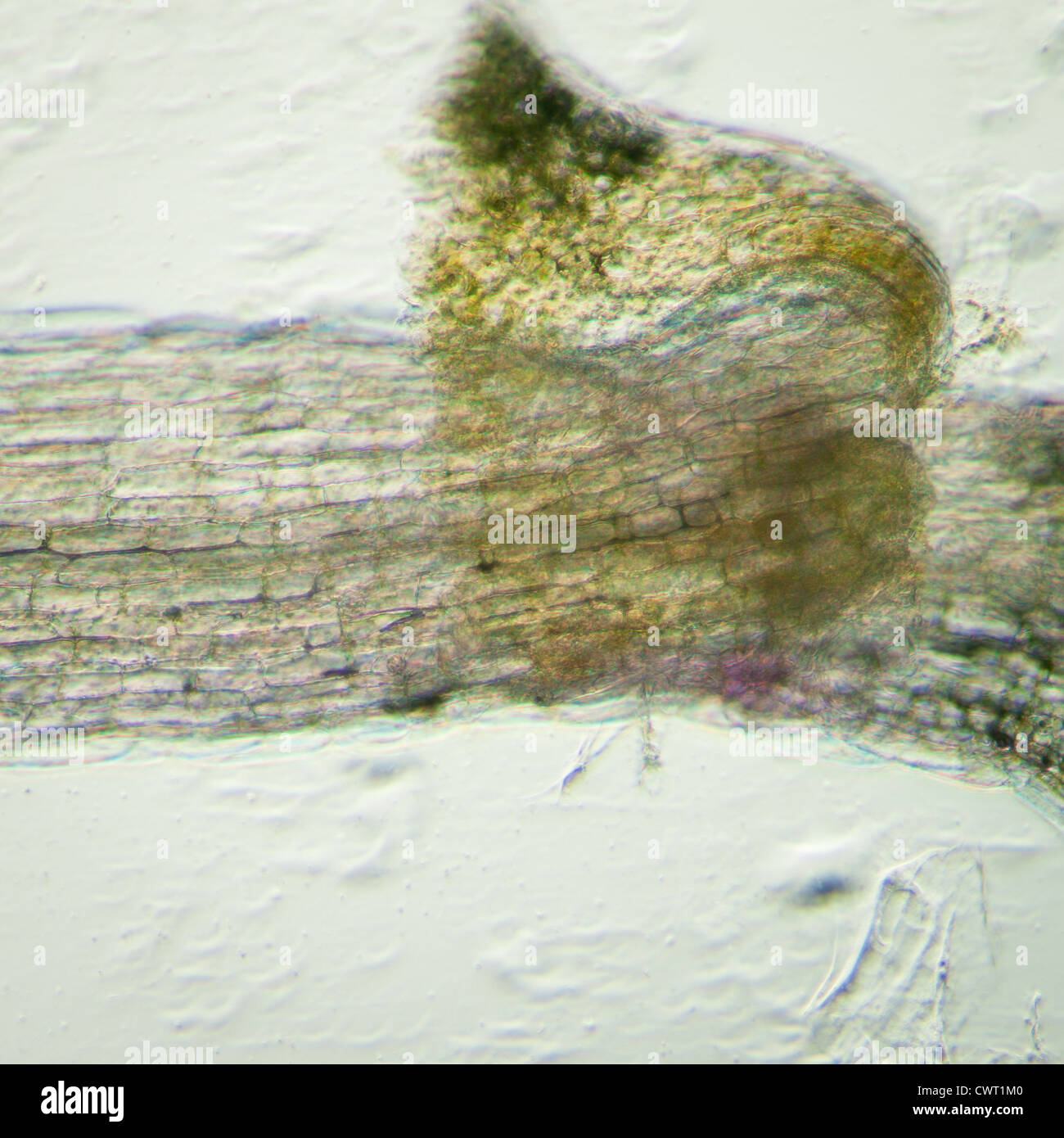 science botany micrograph plant arabidopsis thaliana root tissue micro Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/stock-photo-science-botany-micrograph-plant-arabidopsis-thaliana-root-tissue-micro-50315328.html
science botany micrograph plant arabidopsis thaliana root tissue micro Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/stock-photo-science-botany-micrograph-plant-arabidopsis-thaliana-root-tissue-micro-50315328.htmlRFCWT1M0–science botany micrograph plant arabidopsis thaliana root tissue micro
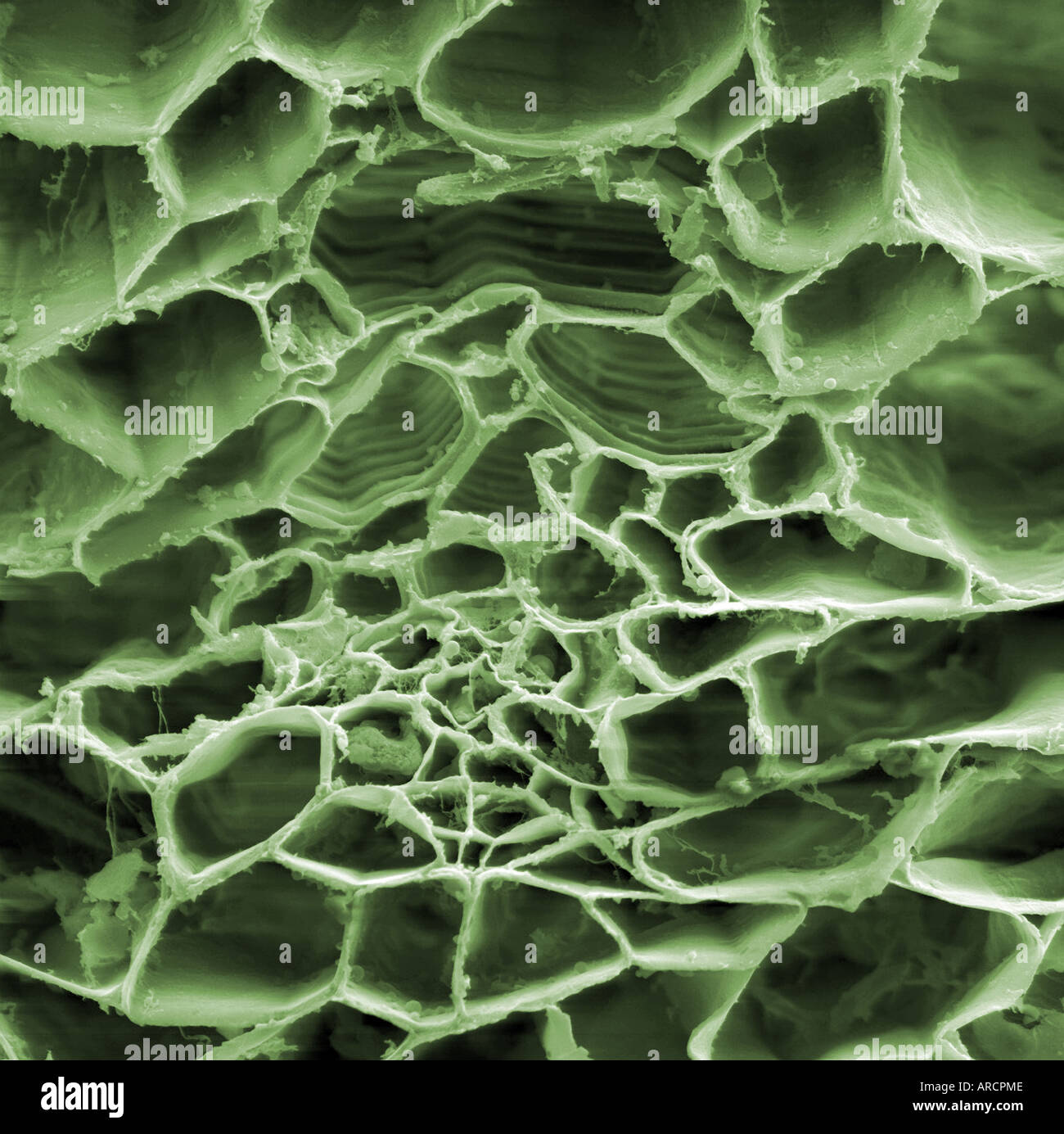 High magnification scanning electron microscope image of a tradescantia (Virginia spiderwort) Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/high-magnification-scanning-electron-microscope-image-of-a-tradescantia-image9150669.html
High magnification scanning electron microscope image of a tradescantia (Virginia spiderwort) Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/high-magnification-scanning-electron-microscope-image-of-a-tradescantia-image9150669.htmlRMARCPME–High magnification scanning electron microscope image of a tradescantia (Virginia spiderwort)
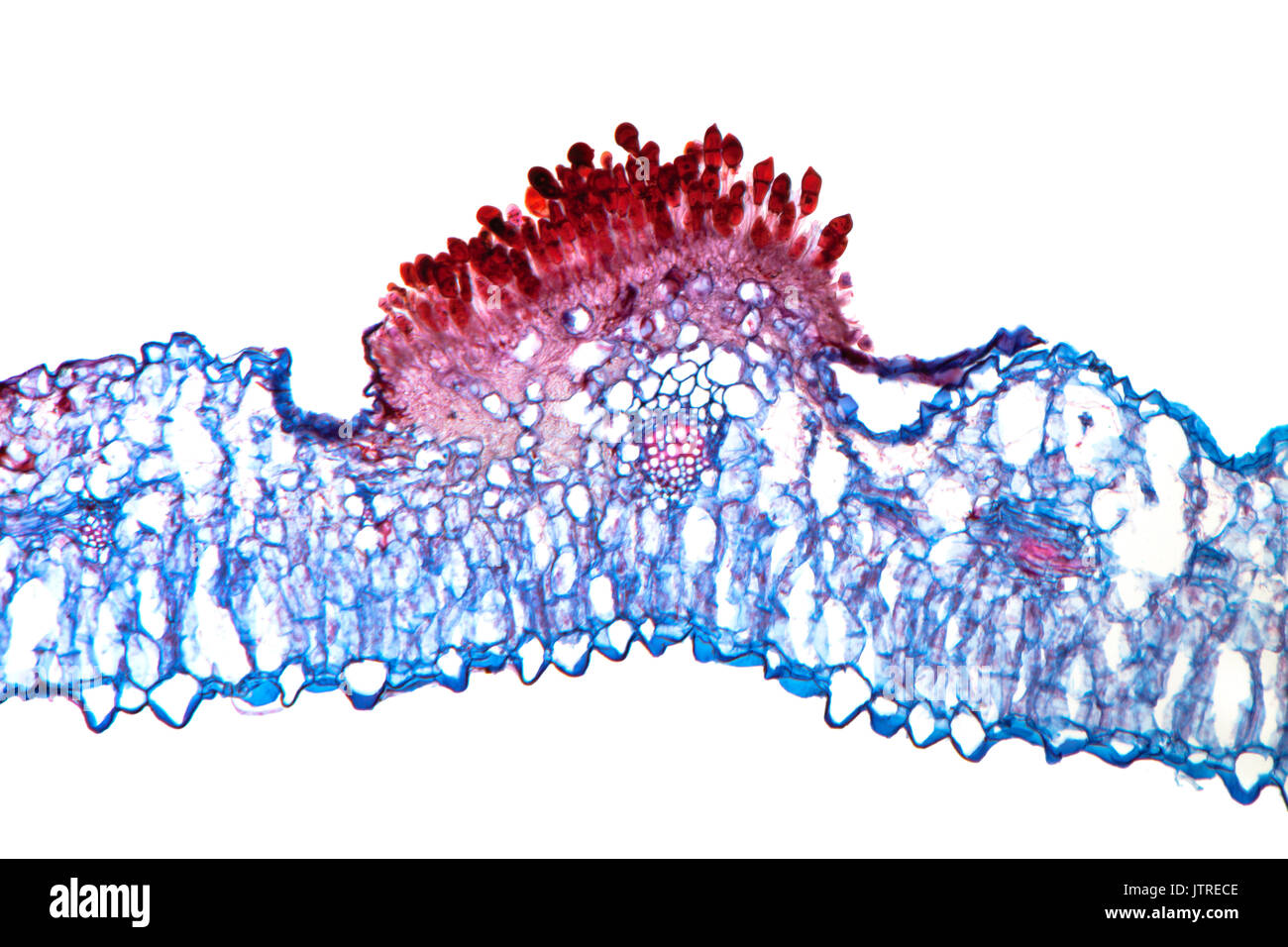 Snapdragon rust, Puccinia antirrhini fungus on antirrhinum leaf, brightfield photomicrograph, TS Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/snapdragon-rust-puccinia-antirrhini-fungus-on-antirrhinum-leaf-brightfield-image152950910.html
Snapdragon rust, Puccinia antirrhini fungus on antirrhinum leaf, brightfield photomicrograph, TS Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/snapdragon-rust-puccinia-antirrhini-fungus-on-antirrhinum-leaf-brightfield-image152950910.htmlRMJTRECE–Snapdragon rust, Puccinia antirrhini fungus on antirrhinum leaf, brightfield photomicrograph, TS
 biological cell isolated on whithe background microscope 3D Illustration Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/biological-cell-isolated-on-whithe-background-microscope-3d-illustration-image208953306.html
biological cell isolated on whithe background microscope 3D Illustration Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/biological-cell-isolated-on-whithe-background-microscope-3d-illustration-image208953306.htmlRFP3XJ22–biological cell isolated on whithe background microscope 3D Illustration
 biological cell isolated on whithe background microscope 3D Illustration Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/biological-cell-isolated-on-whithe-background-microscope-3d-illustration-image208953319.html
biological cell isolated on whithe background microscope 3D Illustration Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/biological-cell-isolated-on-whithe-background-microscope-3d-illustration-image208953319.htmlRFP3XJ2F–biological cell isolated on whithe background microscope 3D Illustration
 Pine Leaf Stomata Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/stock-photo-pine-leaf-stomata-134943696.html
Pine Leaf Stomata Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/stock-photo-pine-leaf-stomata-134943696.htmlRMHRF61M–Pine Leaf Stomata
 Light photomicrograph of Leaf transversal section seen through microscope Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/light-photomicrograph-of-leaf-transversal-section-seen-through-microscope-image179104200.html
Light photomicrograph of Leaf transversal section seen through microscope Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/light-photomicrograph-of-leaf-transversal-section-seen-through-microscope-image179104200.htmlRFMBAW6G–Light photomicrograph of Leaf transversal section seen through microscope
 Longitudinal section portion fir Abies sp needle structural elements especially stomata are shown magnification 100x Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/stock-photo-longitudinal-section-portion-fir-abies-sp-needle-structural-elements-56084200.html
Longitudinal section portion fir Abies sp needle structural elements especially stomata are shown magnification 100x Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/stock-photo-longitudinal-section-portion-fir-abies-sp-needle-structural-elements-56084200.htmlRMD76RY4–Longitudinal section portion fir Abies sp needle structural elements especially stomata are shown magnification 100x
 Archive image from page 59 of Cytological methods for the detection,. Cytological methods for the detection, identification, and characterization of orchid viruses and their inclusion bodies cytologicalmetho00kona Year: 1985 -53- « sr > Figure 51. Electron micrograph of virus aggregations in CyMV-infected Cymbidium leaf cell showing the presence of gold particles on their surface. Section is incubated with CyMV antiserum (1/2000 dilution) and labelled with protein A-gold. Bar = 260 nm, Figure 52. Electron micrograph of virus-like structures in the same antiserum-treated section as in F Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/archive-image-from-page-59-of-cytological-methods-for-the-detection-cytological-methods-for-the-detection-identification-and-characterization-of-orchid-viruses-and-their-inclusion-bodies-cytologicalmetho00kona-year-1985-53-sr-gt-figure-51-electron-micrograph-of-virus-aggregations-in-cymv-infected-cymbidium-leaf-cell-showing-the-presence-of-gold-particles-on-their-surface-section-is-incubated-with-cymv-antiserum-12000-dilution-and-labelled-with-protein-a-gold-bar-=-260-nm-figure-52-electron-micrograph-of-virus-like-structures-in-the-same-antiserum-treated-section-as-in-f-image259287450.html
Archive image from page 59 of Cytological methods for the detection,. Cytological methods for the detection, identification, and characterization of orchid viruses and their inclusion bodies cytologicalmetho00kona Year: 1985 -53- « sr > Figure 51. Electron micrograph of virus aggregations in CyMV-infected Cymbidium leaf cell showing the presence of gold particles on their surface. Section is incubated with CyMV antiserum (1/2000 dilution) and labelled with protein A-gold. Bar = 260 nm, Figure 52. Electron micrograph of virus-like structures in the same antiserum-treated section as in F Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/archive-image-from-page-59-of-cytological-methods-for-the-detection-cytological-methods-for-the-detection-identification-and-characterization-of-orchid-viruses-and-their-inclusion-bodies-cytologicalmetho00kona-year-1985-53-sr-gt-figure-51-electron-micrograph-of-virus-aggregations-in-cymv-infected-cymbidium-leaf-cell-showing-the-presence-of-gold-particles-on-their-surface-section-is-incubated-with-cymv-antiserum-12000-dilution-and-labelled-with-protein-a-gold-bar-=-260-nm-figure-52-electron-micrograph-of-virus-like-structures-in-the-same-antiserum-treated-section-as-in-f-image259287450.htmlRMW1RFP2–Archive image from page 59 of Cytological methods for the detection,. Cytological methods for the detection, identification, and characterization of orchid viruses and their inclusion bodies cytologicalmetho00kona Year: 1985 -53- « sr > Figure 51. Electron micrograph of virus aggregations in CyMV-infected Cymbidium leaf cell showing the presence of gold particles on their surface. Section is incubated with CyMV antiserum (1/2000 dilution) and labelled with protein A-gold. Bar = 260 nm, Figure 52. Electron micrograph of virus-like structures in the same antiserum-treated section as in F
 Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/-image225982099.html
Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/-image225982099.htmlRFR3JAD7–
 . Cytological methods for the detection, identification, and characterization of orchid viruses and their inclusion bodies. Orchids; Virus diseases of plants. -55-. Figure 55. Electron micrograph of virus particles distributed within the cytoplasm of CMV- infected Phalaenopsis leaf cells. Section was incubated with CMV antiserum (1/1000 dilution) and labelled with protein A-gold Pd:Plasmodesma. CW:Cell wall. Bar = 350 nm. Figure 56. Same tissue and treatments as in Fig. 55, except that incubation was with normal serum (1/500 dilution). Note the background of nonspecific reaction (arrows). CWrC Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/cytological-methods-for-the-detection-identification-and-characterization-of-orchid-viruses-and-their-inclusion-bodies-orchids-virus-diseases-of-plants-55-figure-55-electron-micrograph-of-virus-particles-distributed-within-the-cytoplasm-of-cmv-infected-phalaenopsis-leaf-cells-section-was-incubated-with-cmv-antiserum-11000-dilution-and-labelled-with-protein-a-gold-pdplasmodesma-cwcell-wall-bar-=-350-nm-figure-56-same-tissue-and-treatments-as-in-fig-55-except-that-incubation-was-with-normal-serum-1500-dilution-note-the-background-of-nonspecific-reaction-arrows-cwrc-image216162181.html
. Cytological methods for the detection, identification, and characterization of orchid viruses and their inclusion bodies. Orchids; Virus diseases of plants. -55-. Figure 55. Electron micrograph of virus particles distributed within the cytoplasm of CMV- infected Phalaenopsis leaf cells. Section was incubated with CMV antiserum (1/1000 dilution) and labelled with protein A-gold Pd:Plasmodesma. CW:Cell wall. Bar = 350 nm. Figure 56. Same tissue and treatments as in Fig. 55, except that incubation was with normal serum (1/500 dilution). Note the background of nonspecific reaction (arrows). CWrC Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/cytological-methods-for-the-detection-identification-and-characterization-of-orchid-viruses-and-their-inclusion-bodies-orchids-virus-diseases-of-plants-55-figure-55-electron-micrograph-of-virus-particles-distributed-within-the-cytoplasm-of-cmv-infected-phalaenopsis-leaf-cells-section-was-incubated-with-cmv-antiserum-11000-dilution-and-labelled-with-protein-a-gold-pdplasmodesma-cwcell-wall-bar-=-350-nm-figure-56-same-tissue-and-treatments-as-in-fig-55-except-that-incubation-was-with-normal-serum-1500-dilution-note-the-background-of-nonspecific-reaction-arrows-cwrc-image216162181.htmlRMPFK11W–. Cytological methods for the detection, identification, and characterization of orchid viruses and their inclusion bodies. Orchids; Virus diseases of plants. -55-. Figure 55. Electron micrograph of virus particles distributed within the cytoplasm of CMV- infected Phalaenopsis leaf cells. Section was incubated with CMV antiserum (1/1000 dilution) and labelled with protein A-gold Pd:Plasmodesma. CW:Cell wall. Bar = 350 nm. Figure 56. Same tissue and treatments as in Fig. 55, except that incubation was with normal serum (1/500 dilution). Note the background of nonspecific reaction (arrows). CWrC
 . Cytological methods for the detection, identification, and characterization of orchid viruses and their inclusion bodies. Orchids; Virus diseases of plants. -53- « * sr >. Figure 51. Electron micrograph of virus aggregations in CyMV-infected Cymbidium leaf cell showing the presence of gold particles on their surface. Section is incubated with CyMV antiserum (1/2000 dilution) and labelled with protein A-gold. Bar = 260 nm, Figure 52. Electron micrograph of virus-like structures in the same antiserum-treated section as in Fig. 51, but showing no specific reaction. Same treatment as in Fig. Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/cytological-methods-for-the-detection-identification-and-characterization-of-orchid-viruses-and-their-inclusion-bodies-orchids-virus-diseases-of-plants-53-sr-gt-figure-51-electron-micrograph-of-virus-aggregations-in-cymv-infected-cymbidium-leaf-cell-showing-the-presence-of-gold-particles-on-their-surface-section-is-incubated-with-cymv-antiserum-12000-dilution-and-labelled-with-protein-a-gold-bar-=-260-nm-figure-52-electron-micrograph-of-virus-like-structures-in-the-same-antiserum-treated-section-as-in-fig-51-but-showing-no-specific-reaction-same-treatment-as-in-fig-image231779182.html
. Cytological methods for the detection, identification, and characterization of orchid viruses and their inclusion bodies. Orchids; Virus diseases of plants. -53- « * sr >. Figure 51. Electron micrograph of virus aggregations in CyMV-infected Cymbidium leaf cell showing the presence of gold particles on their surface. Section is incubated with CyMV antiserum (1/2000 dilution) and labelled with protein A-gold. Bar = 260 nm, Figure 52. Electron micrograph of virus-like structures in the same antiserum-treated section as in Fig. 51, but showing no specific reaction. Same treatment as in Fig. Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/cytological-methods-for-the-detection-identification-and-characterization-of-orchid-viruses-and-their-inclusion-bodies-orchids-virus-diseases-of-plants-53-sr-gt-figure-51-electron-micrograph-of-virus-aggregations-in-cymv-infected-cymbidium-leaf-cell-showing-the-presence-of-gold-particles-on-their-surface-section-is-incubated-with-cymv-antiserum-12000-dilution-and-labelled-with-protein-a-gold-bar-=-260-nm-figure-52-electron-micrograph-of-virus-like-structures-in-the-same-antiserum-treated-section-as-in-fig-51-but-showing-no-specific-reaction-same-treatment-as-in-fig-image231779182.htmlRMRD2CKX–. Cytological methods for the detection, identification, and characterization of orchid viruses and their inclusion bodies. Orchids; Virus diseases of plants. -53- « * sr >. Figure 51. Electron micrograph of virus aggregations in CyMV-infected Cymbidium leaf cell showing the presence of gold particles on their surface. Section is incubated with CyMV antiserum (1/2000 dilution) and labelled with protein A-gold. Bar = 260 nm, Figure 52. Electron micrograph of virus-like structures in the same antiserum-treated section as in Fig. 51, but showing no specific reaction. Same treatment as in Fig.
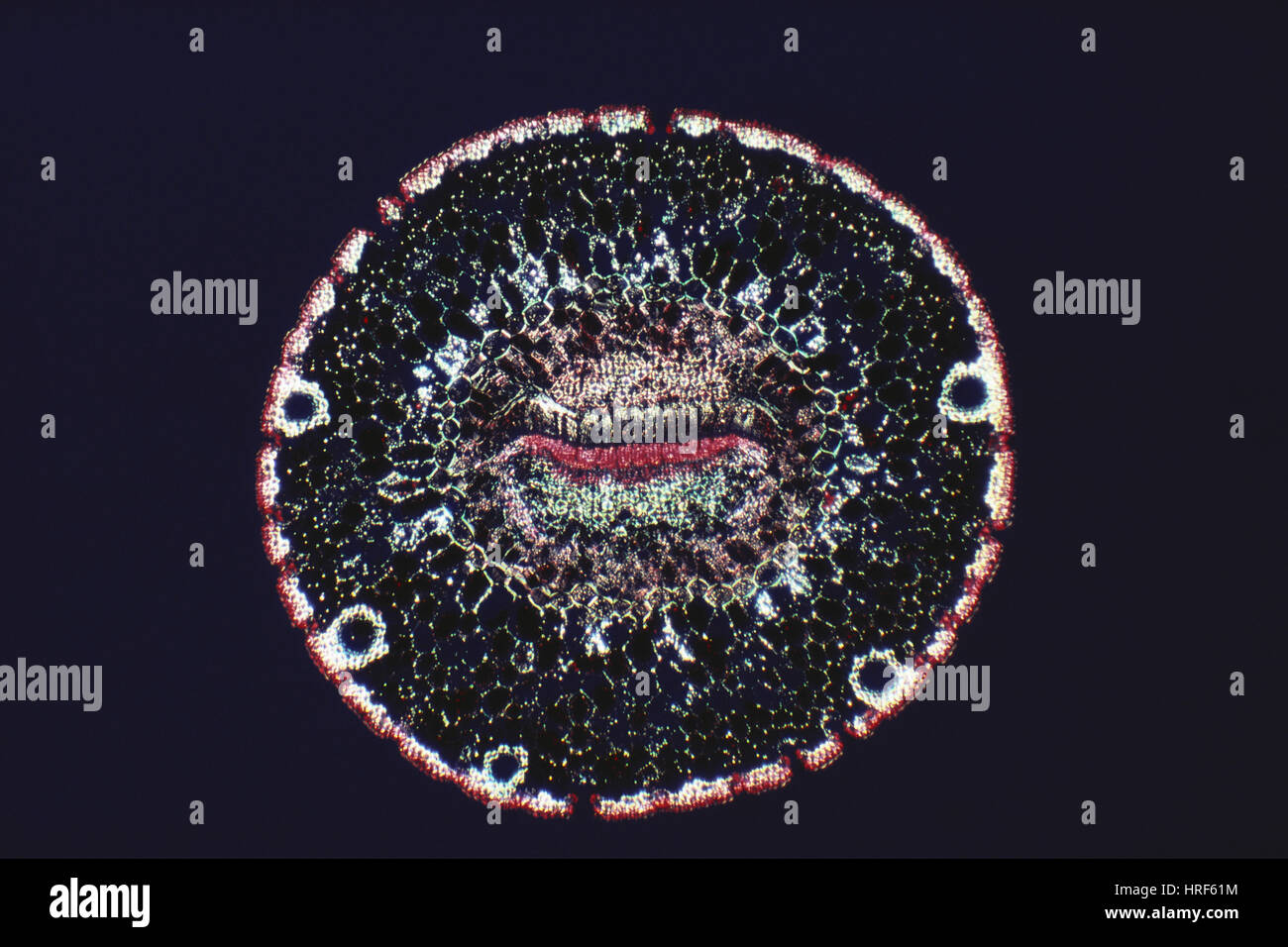
 Cross section of monocot stem, light micrograph Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/cross-section-of-monocot-stem-light-micrograph-image436756303.html
Cross section of monocot stem, light micrograph Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/cross-section-of-monocot-stem-light-micrograph-image436756303.htmlRF2GAFY3B–Cross section of monocot stem, light micrograph
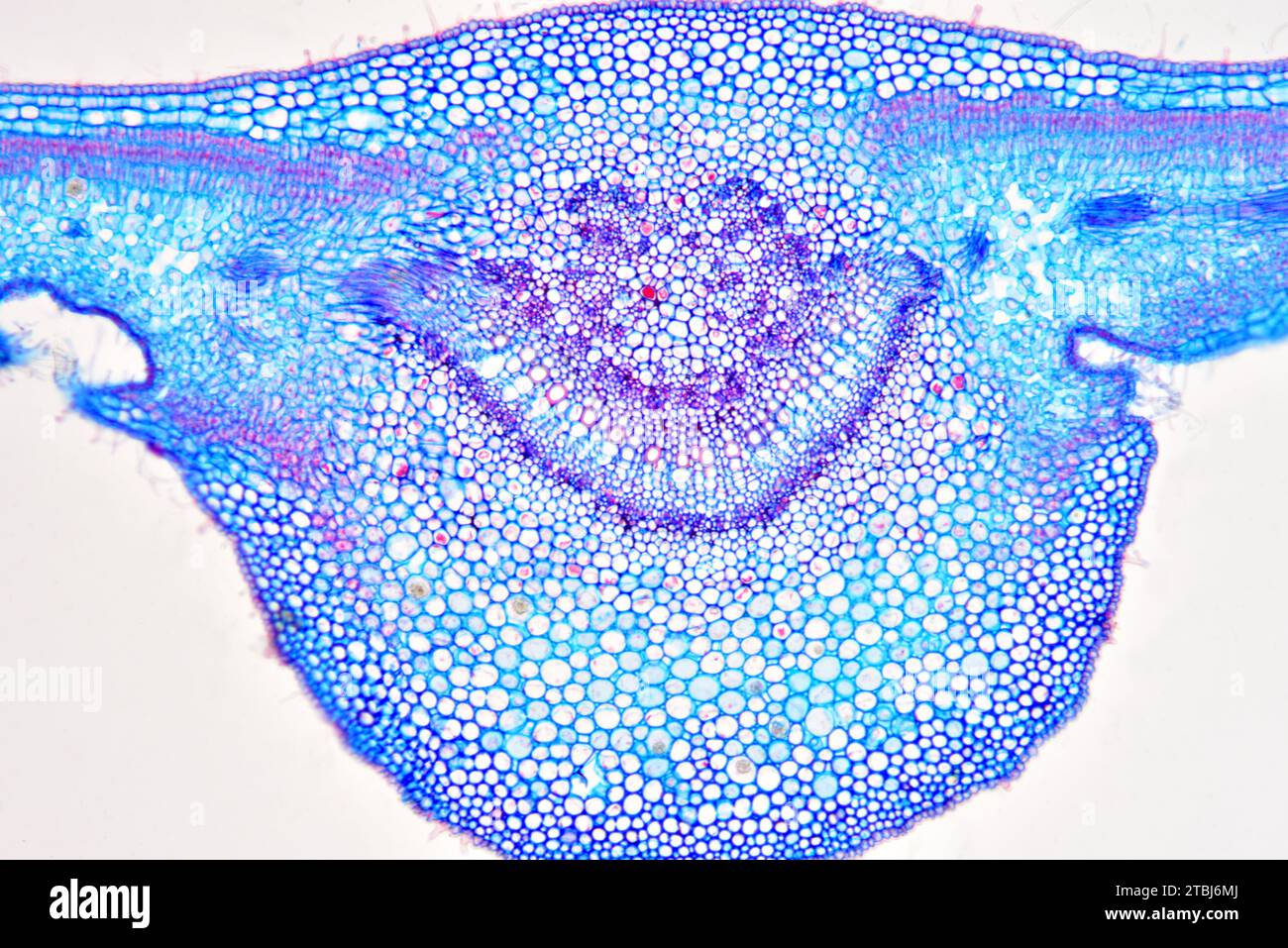 Leaf cross section of Nerium oleander showing epidermis, palisade mesophyll, spongy mesophyll, vascular bundle, phloem, xylem, collenchyma and stomata Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/leaf-cross-section-of-nerium-oleander-showing-epidermis-palisade-mesophyll-spongy-mesophyll-vascular-bundle-phloem-xylem-collenchyma-and-stomata-image575103778.html
Leaf cross section of Nerium oleander showing epidermis, palisade mesophyll, spongy mesophyll, vascular bundle, phloem, xylem, collenchyma and stomata Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/leaf-cross-section-of-nerium-oleander-showing-epidermis-palisade-mesophyll-spongy-mesophyll-vascular-bundle-phloem-xylem-collenchyma-and-stomata-image575103778.htmlRF2TBJ6MJ–Leaf cross section of Nerium oleander showing epidermis, palisade mesophyll, spongy mesophyll, vascular bundle, phloem, xylem, collenchyma and stomata
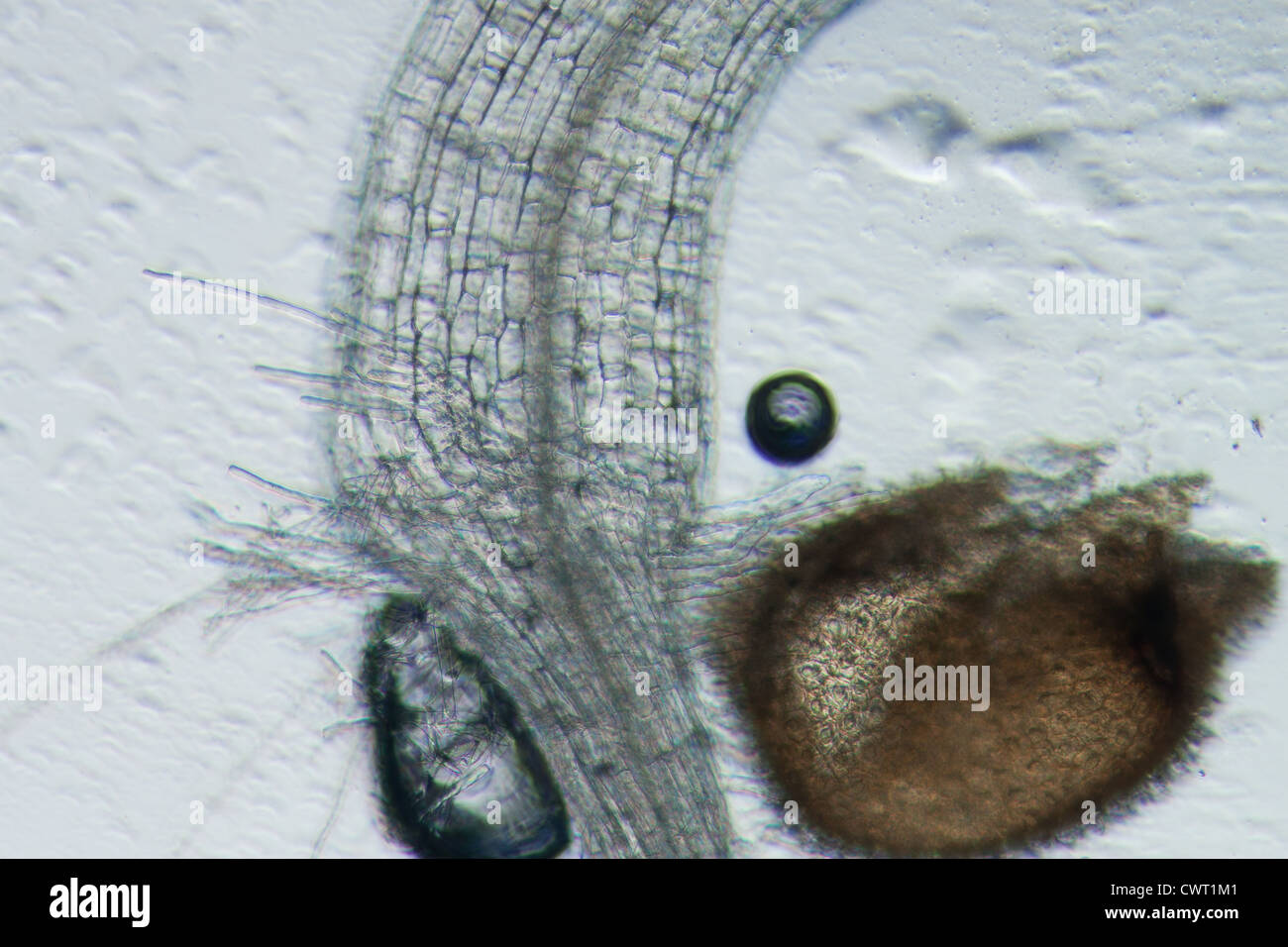 science botany micrograph plant arabidopsis thaliana root tissue micro Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/stock-photo-science-botany-micrograph-plant-arabidopsis-thaliana-root-tissue-micro-50315329.html
science botany micrograph plant arabidopsis thaliana root tissue micro Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/stock-photo-science-botany-micrograph-plant-arabidopsis-thaliana-root-tissue-micro-50315329.htmlRFCWT1M1–science botany micrograph plant arabidopsis thaliana root tissue micro
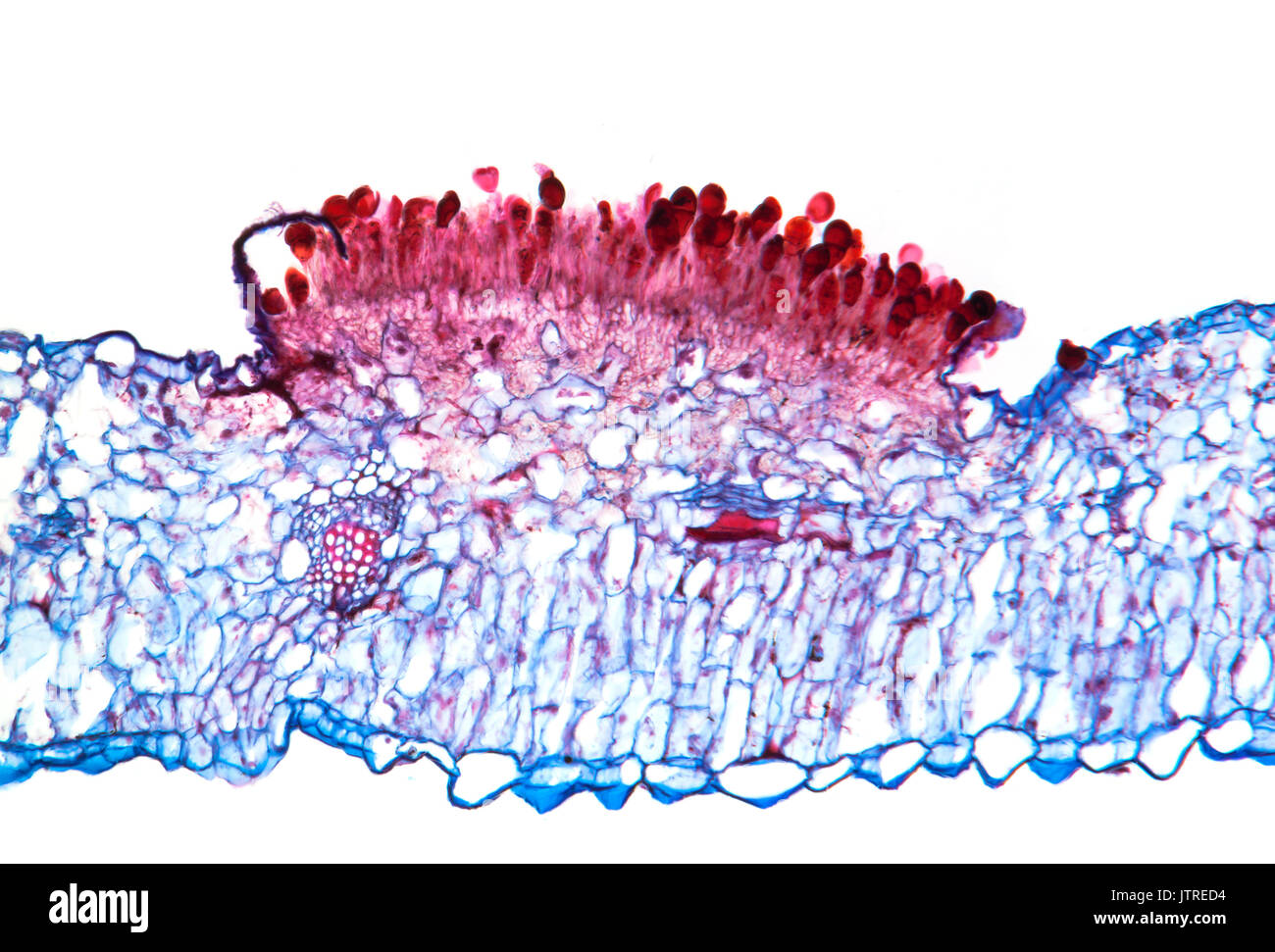 Snapdragon rust, Puccinia antirrhini fungus on antirrhinum leaf, brightfield photomicrograph, TS Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/snapdragon-rust-puccinia-antirrhini-fungus-on-antirrhinum-leaf-brightfield-image152950928.html
Snapdragon rust, Puccinia antirrhini fungus on antirrhinum leaf, brightfield photomicrograph, TS Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/snapdragon-rust-puccinia-antirrhini-fungus-on-antirrhinum-leaf-brightfield-image152950928.htmlRMJTRED4–Snapdragon rust, Puccinia antirrhini fungus on antirrhinum leaf, brightfield photomicrograph, TS
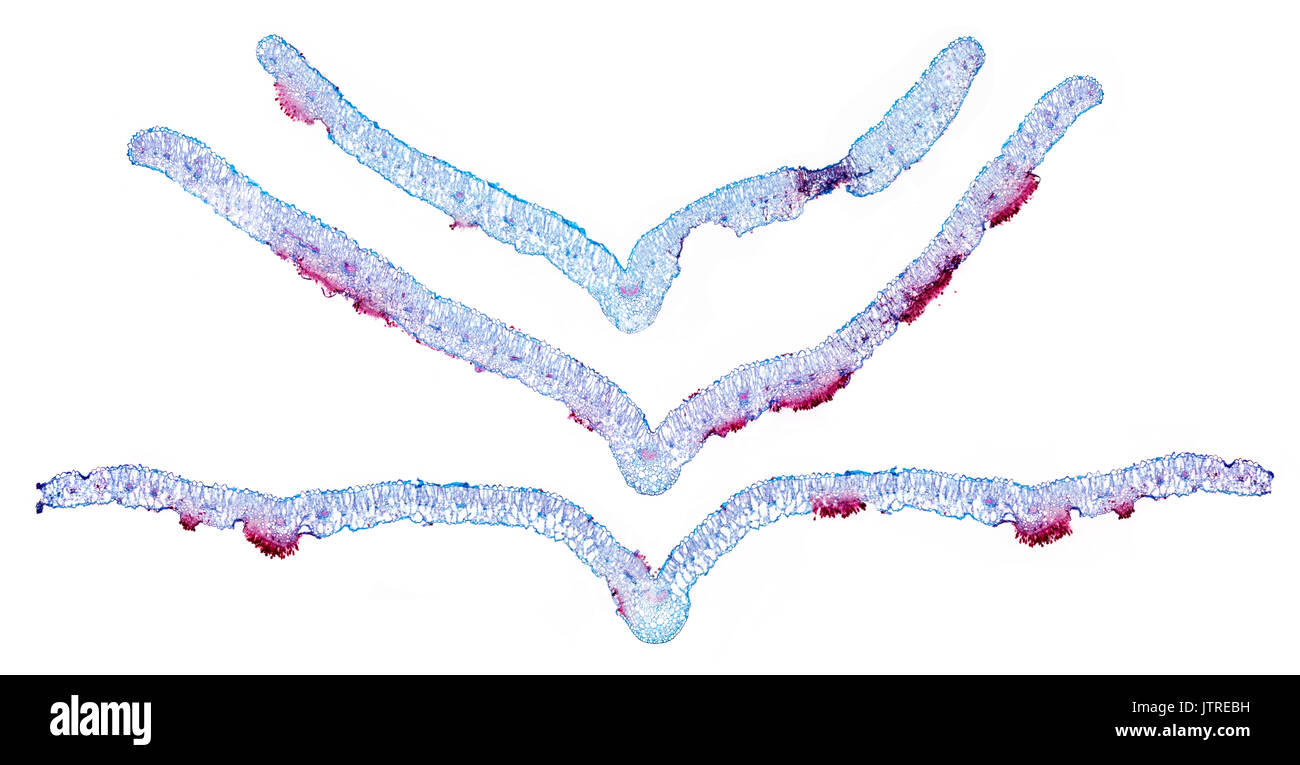 Snapdragon rust, Puccinia antirrhini fungus on antirrhinum leaf, brightfield photomicrograph, TS Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/snapdragon-rust-puccinia-antirrhini-fungus-on-antirrhinum-leaf-brightfield-image152950885.html
Snapdragon rust, Puccinia antirrhini fungus on antirrhinum leaf, brightfield photomicrograph, TS Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/snapdragon-rust-puccinia-antirrhini-fungus-on-antirrhinum-leaf-brightfield-image152950885.htmlRMJTREBH–Snapdragon rust, Puccinia antirrhini fungus on antirrhinum leaf, brightfield photomicrograph, TS
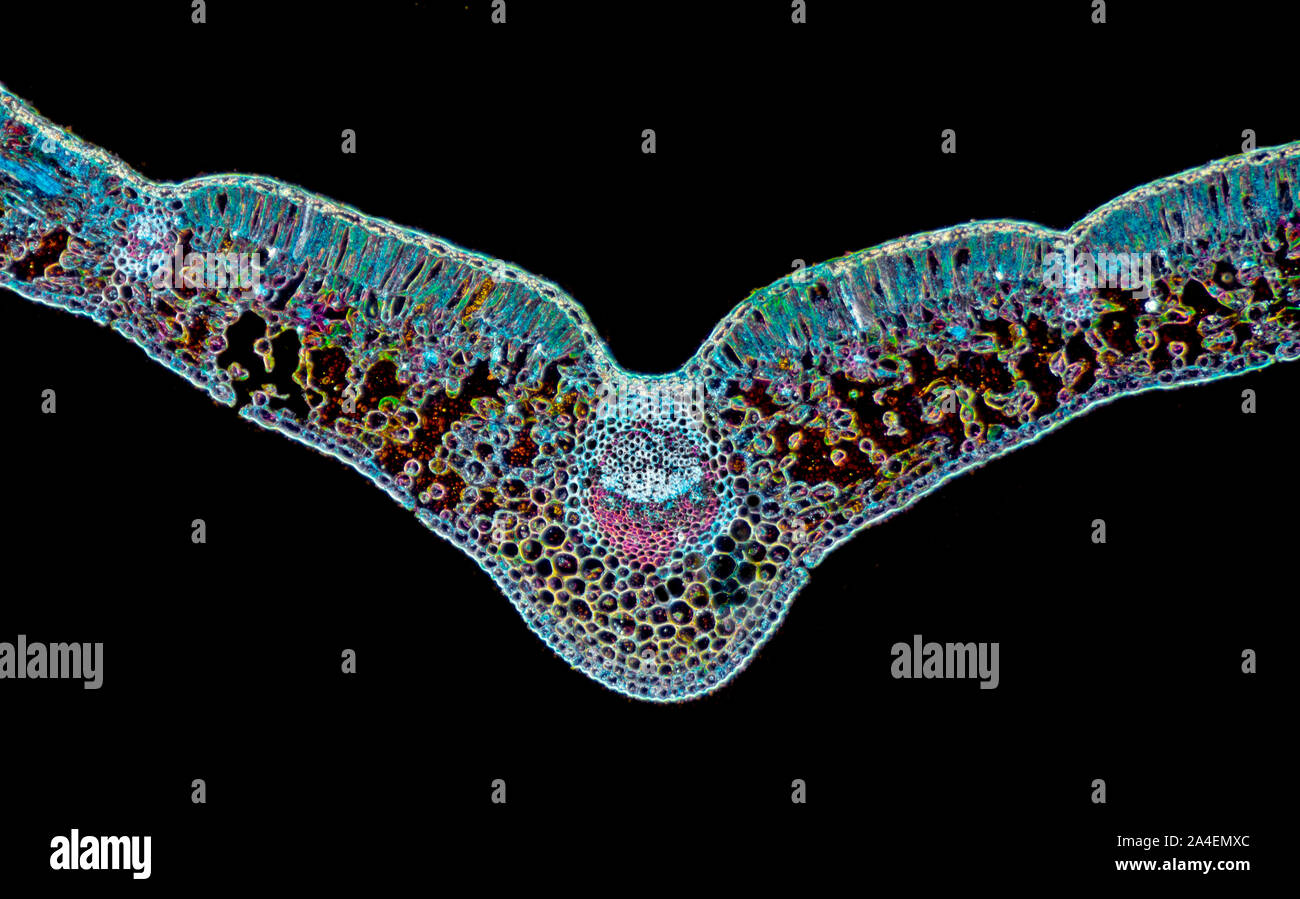 Helleborus niger, Christmas Rose, stained leaf section, TS. Darkfield photomicrograph Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/helleborus-niger-christmas-rose-stained-leaf-section-ts-darkfield-photomicrograph-image329779364.html
Helleborus niger, Christmas Rose, stained leaf section, TS. Darkfield photomicrograph Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/helleborus-niger-christmas-rose-stained-leaf-section-ts-darkfield-photomicrograph-image329779364.htmlRM2A4EMXC–Helleborus niger, Christmas Rose, stained leaf section, TS. Darkfield photomicrograph
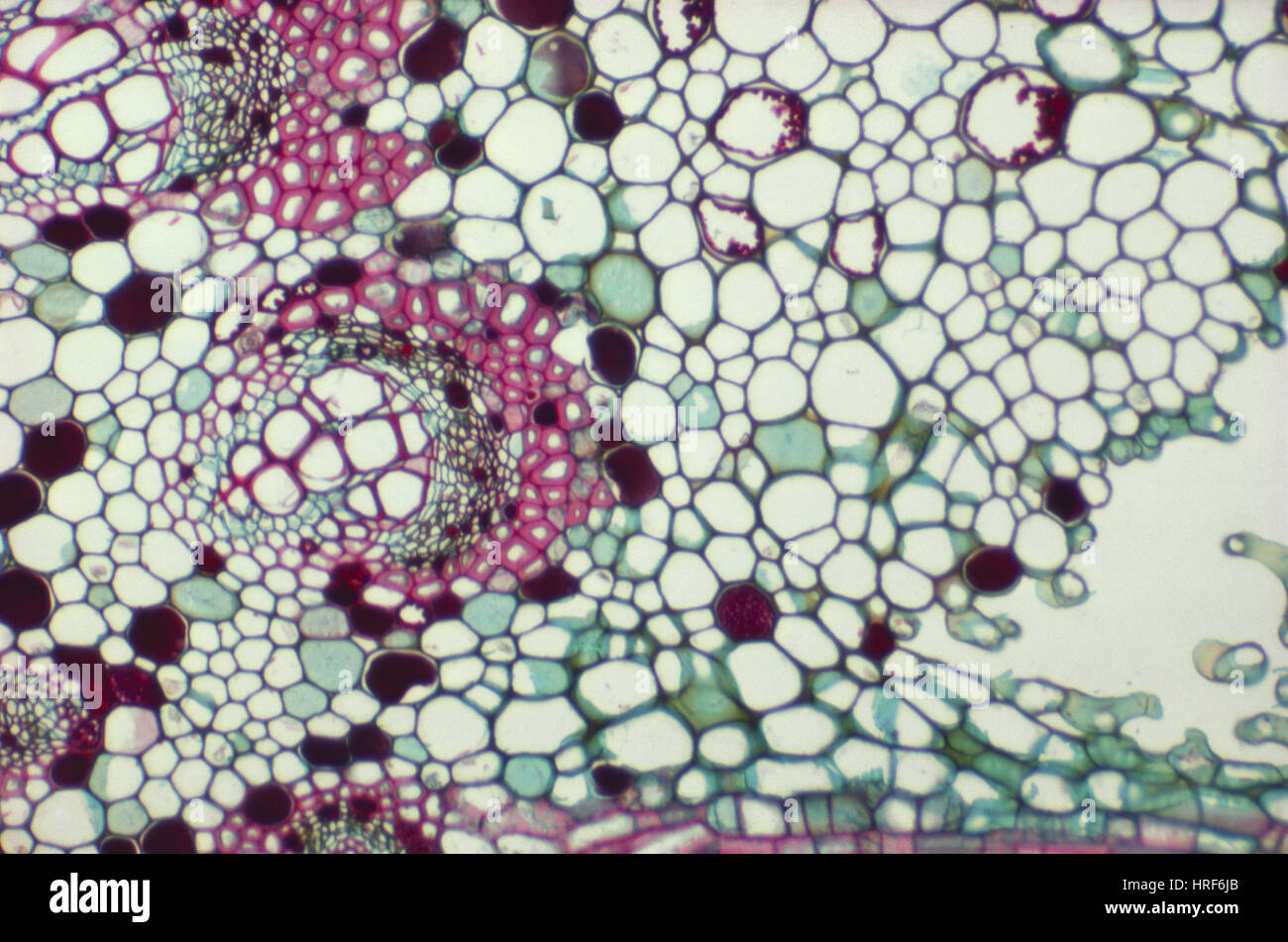 LM of Fig Leaf Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/stock-photo-lm-of-fig-leaf-134944163.html
LM of Fig Leaf Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/stock-photo-lm-of-fig-leaf-134944163.htmlRMHRF6JB–LM of Fig Leaf
 Brightfield photomicrograph, Sycamore leaf section TS showing central vein and cell structure Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/stock-photo-brightfield-photomicrograph-sycamore-leaf-section-ts-showing-central-105720047.html
Brightfield photomicrograph, Sycamore leaf section TS showing central vein and cell structure Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/stock-photo-brightfield-photomicrograph-sycamore-leaf-section-ts-showing-central-105720047.htmlRMG3YXYY–Brightfield photomicrograph, Sycamore leaf section TS showing central vein and cell structure
 Chara algae showing stem oogonium round structure antheridiium pointed structures are new leaves magnification 40x Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/stock-photo-chara-algae-showing-stem-oogonium-round-structure-antheridiium-pointed-56084159.html
Chara algae showing stem oogonium round structure antheridiium pointed structures are new leaves magnification 40x Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/stock-photo-chara-algae-showing-stem-oogonium-round-structure-antheridiium-pointed-56084159.htmlRMD76RWK–Chara algae showing stem oogonium round structure antheridiium pointed structures are new leaves magnification 40x
 Datura x candida stem leaf and petiole TS darkfield photomicrograph Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/datura-x-candida-stem-leaf-and-petiole-ts-darkfield-photomicrograph-image239401045.html
Datura x candida stem leaf and petiole TS darkfield photomicrograph Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/datura-x-candida-stem-leaf-and-petiole-ts-darkfield-photomicrograph-image239401045.htmlRMRWDJD9–Datura x candida stem leaf and petiole TS darkfield photomicrograph
 Archive image from page 48 of Cytological methods for the detection,. Cytological methods for the detection, identification, and characterization of orchid viruses and their inclusion bodies cytologicalmetho00kona Year: 1985 â yi i Figure 33. Light micrograph of ORSV-infected cells showing a stacked-plate inclusion (SI, side view) in semithin section (0.5 Mm thick). Section is stained with toluidine blue. Bar = 5 jam. Figure 34. Electron micrograph of ORSV stacked- plate inclusion (side view) in Cattleya leaf cells stained with O/G combination. Bar = 600 nm. Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/archive-image-from-page-48-of-cytological-methods-for-the-detection-cytological-methods-for-the-detection-identification-and-characterization-of-orchid-viruses-and-their-inclusion-bodies-cytologicalmetho00kona-year-1985-yi-i-figure-33-light-micrograph-of-orsv-infected-cells-showing-a-stacked-plate-inclusion-si-side-view-in-semithin-section-05-mm-thick-section-is-stained-with-toluidine-blue-bar-=-5-jam-figure-34-electron-micrograph-of-orsv-stacked-plate-inclusion-side-view-in-cattleya-leaf-cells-stained-with-og-combination-bar-=-600-nm-image259283480.html
Archive image from page 48 of Cytological methods for the detection,. Cytological methods for the detection, identification, and characterization of orchid viruses and their inclusion bodies cytologicalmetho00kona Year: 1985 â yi i Figure 33. Light micrograph of ORSV-infected cells showing a stacked-plate inclusion (SI, side view) in semithin section (0.5 Mm thick). Section is stained with toluidine blue. Bar = 5 jam. Figure 34. Electron micrograph of ORSV stacked- plate inclusion (side view) in Cattleya leaf cells stained with O/G combination. Bar = 600 nm. Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/archive-image-from-page-48-of-cytological-methods-for-the-detection-cytological-methods-for-the-detection-identification-and-characterization-of-orchid-viruses-and-their-inclusion-bodies-cytologicalmetho00kona-year-1985-yi-i-figure-33-light-micrograph-of-orsv-infected-cells-showing-a-stacked-plate-inclusion-si-side-view-in-semithin-section-05-mm-thick-section-is-stained-with-toluidine-blue-bar-=-5-jam-figure-34-electron-micrograph-of-orsv-stacked-plate-inclusion-side-view-in-cattleya-leaf-cells-stained-with-og-combination-bar-=-600-nm-image259283480.htmlRMW1RAM8–Archive image from page 48 of Cytological methods for the detection,. Cytological methods for the detection, identification, and characterization of orchid viruses and their inclusion bodies cytologicalmetho00kona Year: 1985 â yi i Figure 33. Light micrograph of ORSV-infected cells showing a stacked-plate inclusion (SI, side view) in semithin section (0.5 Mm thick). Section is stained with toluidine blue. Bar = 5 jam. Figure 34. Electron micrograph of ORSV stacked- plate inclusion (side view) in Cattleya leaf cells stained with O/G combination. Bar = 600 nm.
 . Cytological methods for the detection, identification, and characterization of orchid viruses and their inclusion bodies. Orchids; Virus diseases of plants. â yi i. Figure 33. Light micrograph of ORSV-infected cells showing a stacked-plate inclusion (SI, side view) in semithin section (0.5 Mm thick). Section is stained with toluidine blue. Bar = 5 jam. Figure 34. Electron micrograph of ORSV stacked- plate inclusion (side view) in Cattleya leaf cells stained with O/G combination. Bar = 600 nm.. Please note that these images are extracted from scanned page images that may have been digitally e Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/cytological-methods-for-the-detection-identification-and-characterization-of-orchid-viruses-and-their-inclusion-bodies-orchids-virus-diseases-of-plants-yi-i-figure-33-light-micrograph-of-orsv-infected-cells-showing-a-stacked-plate-inclusion-si-side-view-in-semithin-section-05-mm-thick-section-is-stained-with-toluidine-blue-bar-=-5-jam-figure-34-electron-micrograph-of-orsv-stacked-plate-inclusion-side-view-in-cattleya-leaf-cells-stained-with-og-combination-bar-=-600-nm-please-note-that-these-images-are-extracted-from-scanned-page-images-that-may-have-been-digitally-e-image231811256.html
. Cytological methods for the detection, identification, and characterization of orchid viruses and their inclusion bodies. Orchids; Virus diseases of plants. â yi i. Figure 33. Light micrograph of ORSV-infected cells showing a stacked-plate inclusion (SI, side view) in semithin section (0.5 Mm thick). Section is stained with toluidine blue. Bar = 5 jam. Figure 34. Electron micrograph of ORSV stacked- plate inclusion (side view) in Cattleya leaf cells stained with O/G combination. Bar = 600 nm.. Please note that these images are extracted from scanned page images that may have been digitally e Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/cytological-methods-for-the-detection-identification-and-characterization-of-orchid-viruses-and-their-inclusion-bodies-orchids-virus-diseases-of-plants-yi-i-figure-33-light-micrograph-of-orsv-infected-cells-showing-a-stacked-plate-inclusion-si-side-view-in-semithin-section-05-mm-thick-section-is-stained-with-toluidine-blue-bar-=-5-jam-figure-34-electron-micrograph-of-orsv-stacked-plate-inclusion-side-view-in-cattleya-leaf-cells-stained-with-og-combination-bar-=-600-nm-please-note-that-these-images-are-extracted-from-scanned-page-images-that-may-have-been-digitally-e-image231811256.htmlRMRD3WHC–. Cytological methods for the detection, identification, and characterization of orchid viruses and their inclusion bodies. Orchids; Virus diseases of plants. â yi i. Figure 33. Light micrograph of ORSV-infected cells showing a stacked-plate inclusion (SI, side view) in semithin section (0.5 Mm thick). Section is stained with toluidine blue. Bar = 5 jam. Figure 34. Electron micrograph of ORSV stacked- plate inclusion (side view) in Cattleya leaf cells stained with O/G combination. Bar = 600 nm.. Please note that these images are extracted from scanned page images that may have been digitally e
 . Cytological methods for the detection, identification, and characterization of orchid viruses and their inclusion bodies. Orchids; Virus diseases of plants. â yi i. Figure 33. Light micrograph of ORSV-infected cells showing a stacked-plate inclusion (SI, side view) in semithin section (0.5 Mm thick). Section is stained with toluidine blue. Bar = 5 jam. Figure 34. Electron micrograph of ORSV stacked- plate inclusion (side view) in Cattleya leaf cells stained with O/G combination. Bar = 600 nm.. Please note that these images are extracted from scanned page images that may have been digitally e Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/cytological-methods-for-the-detection-identification-and-characterization-of-orchid-viruses-and-their-inclusion-bodies-orchids-virus-diseases-of-plants-yi-i-figure-33-light-micrograph-of-orsv-infected-cells-showing-a-stacked-plate-inclusion-si-side-view-in-semithin-section-05-mm-thick-section-is-stained-with-toluidine-blue-bar-=-5-jam-figure-34-electron-micrograph-of-orsv-stacked-plate-inclusion-side-view-in-cattleya-leaf-cells-stained-with-og-combination-bar-=-600-nm-please-note-that-these-images-are-extracted-from-scanned-page-images-that-may-have-been-digitally-e-image216162284.html
. Cytological methods for the detection, identification, and characterization of orchid viruses and their inclusion bodies. Orchids; Virus diseases of plants. â yi i. Figure 33. Light micrograph of ORSV-infected cells showing a stacked-plate inclusion (SI, side view) in semithin section (0.5 Mm thick). Section is stained with toluidine blue. Bar = 5 jam. Figure 34. Electron micrograph of ORSV stacked- plate inclusion (side view) in Cattleya leaf cells stained with O/G combination. Bar = 600 nm.. Please note that these images are extracted from scanned page images that may have been digitally e Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/cytological-methods-for-the-detection-identification-and-characterization-of-orchid-viruses-and-their-inclusion-bodies-orchids-virus-diseases-of-plants-yi-i-figure-33-light-micrograph-of-orsv-infected-cells-showing-a-stacked-plate-inclusion-si-side-view-in-semithin-section-05-mm-thick-section-is-stained-with-toluidine-blue-bar-=-5-jam-figure-34-electron-micrograph-of-orsv-stacked-plate-inclusion-side-view-in-cattleya-leaf-cells-stained-with-og-combination-bar-=-600-nm-please-note-that-these-images-are-extracted-from-scanned-page-images-that-may-have-been-digitally-e-image216162284.htmlRMPFK15G–. Cytological methods for the detection, identification, and characterization of orchid viruses and their inclusion bodies. Orchids; Virus diseases of plants. â yi i. Figure 33. Light micrograph of ORSV-infected cells showing a stacked-plate inclusion (SI, side view) in semithin section (0.5 Mm thick). Section is stained with toluidine blue. Bar = 5 jam. Figure 34. Electron micrograph of ORSV stacked- plate inclusion (side view) in Cattleya leaf cells stained with O/G combination. Bar = 600 nm.. Please note that these images are extracted from scanned page images that may have been digitally e