Quick filters:
Rosaniline Stock Photos and Images
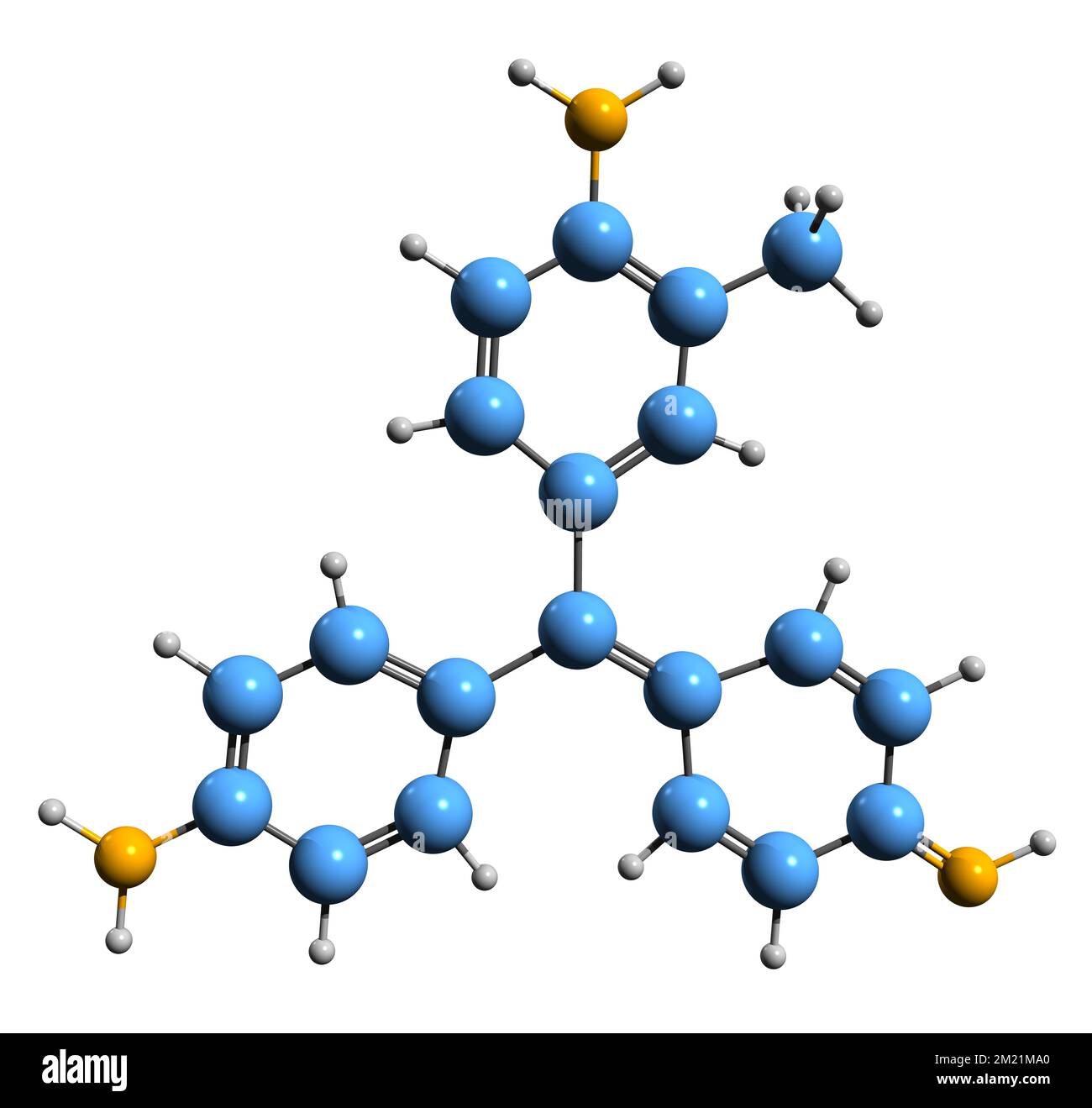 3D image of Fuchsine skeletal formula - molecular chemical structure of rosaniline hydrochloride isolated on white background Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/3d-image-of-fuchsine-skeletal-formula-molecular-chemical-structure-of-rosaniline-hydrochloride-isolated-on-white-background-image500367896.html
3D image of Fuchsine skeletal formula - molecular chemical structure of rosaniline hydrochloride isolated on white background Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/3d-image-of-fuchsine-skeletal-formula-molecular-chemical-structure-of-rosaniline-hydrochloride-isolated-on-white-background-image500367896.htmlRF2M21MA0–3D image of Fuchsine skeletal formula - molecular chemical structure of rosaniline hydrochloride isolated on white background
 Archive image from page 110 of Cytological technique; the principles underlying. Cytological technique; the principles underlying routine methods cytologicaltechn00bake Year: 1960 THE CHEMICAL COMPOSITION OF DYES 91 Beyond auxochromes and chromophores, dyes often possess atoms or groups of atoms that are called modifiers. Thus rosani- NH NH, + Rosaniline line differs from pararosaniline only in the possession of a single methyl group; this modifies the colour, making it very slightly bluer. (Basic fuchsine is a mixture of these two closely related dyes.) The methyl group is here a modifie Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/archive-image-from-page-110-of-cytological-technique-the-principles-underlying-cytological-technique-the-principles-underlying-routine-methods-cytologicaltechn00bake-year-1960-the-chemical-composition-of-dyes-91-beyond-auxochromes-and-chromophores-dyes-often-possess-atoms-or-groups-of-atoms-that-are-called-modifiers-thus-rosani-nh-nh-rosaniline-line-differs-from-pararosaniline-only-in-the-possession-of-a-single-methyl-group-this-modifies-the-colour-making-it-very-slightly-bluer-basic-fuchsine-is-a-mixture-of-these-two-closely-related-dyes-the-methyl-group-is-here-a-modifie-image259447772.html
Archive image from page 110 of Cytological technique; the principles underlying. Cytological technique; the principles underlying routine methods cytologicaltechn00bake Year: 1960 THE CHEMICAL COMPOSITION OF DYES 91 Beyond auxochromes and chromophores, dyes often possess atoms or groups of atoms that are called modifiers. Thus rosani- NH NH, + Rosaniline line differs from pararosaniline only in the possession of a single methyl group; this modifies the colour, making it very slightly bluer. (Basic fuchsine is a mixture of these two closely related dyes.) The methyl group is here a modifie Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/archive-image-from-page-110-of-cytological-technique-the-principles-underlying-cytological-technique-the-principles-underlying-routine-methods-cytologicaltechn00bake-year-1960-the-chemical-composition-of-dyes-91-beyond-auxochromes-and-chromophores-dyes-often-possess-atoms-or-groups-of-atoms-that-are-called-modifiers-thus-rosani-nh-nh-rosaniline-line-differs-from-pararosaniline-only-in-the-possession-of-a-single-methyl-group-this-modifies-the-colour-making-it-very-slightly-bluer-basic-fuchsine-is-a-mixture-of-these-two-closely-related-dyes-the-methyl-group-is-here-a-modifie-image259447772.htmlRMW22T7T–Archive image from page 110 of Cytological technique; the principles underlying. Cytological technique; the principles underlying routine methods cytologicaltechn00bake Year: 1960 THE CHEMICAL COMPOSITION OF DYES 91 Beyond auxochromes and chromophores, dyes often possess atoms or groups of atoms that are called modifiers. Thus rosani- NH NH, + Rosaniline line differs from pararosaniline only in the possession of a single methyl group; this modifies the colour, making it very slightly bluer. (Basic fuchsine is a mixture of these two closely related dyes.) The methyl group is here a modifie
 . Chemistry of dye-stuffs. ub- TRIPHENYLMETHANE DYE-STUFFS. 141 stance, assume the para position towards the methanecarbon. Consequently, the following constitutional formulaeapply to leucaniKne and the rosaniline base respectively :— A ready explanation is thus afforded of the fact, observedsoon after the discovery of fuchsine, namely, that jjure anilinedoes not furnish rosaniline when oxidised, but that the pre-sence of paratoluidine is essential to this reaction, this last-named substance supplying from its methyl group the methanecarbon necessary to the formation of a triphenylmethanederiv Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/chemistry-of-dye-stuffs-ub-triphenylmethane-dye-stuffs-141-stance-assume-the-para-position-towards-the-methanecarbon-consequently-the-following-constitutional-formulaeapply-to-leucanikne-and-the-rosaniline-base-respectively-a-ready-explanation-is-thus-afforded-of-the-fact-observedsoon-after-the-discovery-of-fuchsine-namely-that-jjure-anilinedoes-not-furnish-rosaniline-when-oxidised-but-that-the-pre-sence-of-paratoluidine-is-essential-to-this-reaction-this-last-named-substance-supplying-from-its-methyl-group-the-methanecarbon-necessary-to-the-formation-of-a-triphenylmethanederiv-image336723736.html
. Chemistry of dye-stuffs. ub- TRIPHENYLMETHANE DYE-STUFFS. 141 stance, assume the para position towards the methanecarbon. Consequently, the following constitutional formulaeapply to leucaniKne and the rosaniline base respectively :— A ready explanation is thus afforded of the fact, observedsoon after the discovery of fuchsine, namely, that jjure anilinedoes not furnish rosaniline when oxidised, but that the pre-sence of paratoluidine is essential to this reaction, this last-named substance supplying from its methyl group the methanecarbon necessary to the formation of a triphenylmethanederiv Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/chemistry-of-dye-stuffs-ub-triphenylmethane-dye-stuffs-141-stance-assume-the-para-position-towards-the-methanecarbon-consequently-the-following-constitutional-formulaeapply-to-leucanikne-and-the-rosaniline-base-respectively-a-ready-explanation-is-thus-afforded-of-the-fact-observedsoon-after-the-discovery-of-fuchsine-namely-that-jjure-anilinedoes-not-furnish-rosaniline-when-oxidised-but-that-the-pre-sence-of-paratoluidine-is-essential-to-this-reaction-this-last-named-substance-supplying-from-its-methyl-group-the-methanecarbon-necessary-to-the-formation-of-a-triphenylmethanederiv-image336723736.htmlRM2AFR2FM–. Chemistry of dye-stuffs. ub- TRIPHENYLMETHANE DYE-STUFFS. 141 stance, assume the para position towards the methanecarbon. Consequently, the following constitutional formulaeapply to leucaniKne and the rosaniline base respectively :— A ready explanation is thus afforded of the fact, observedsoon after the discovery of fuchsine, namely, that jjure anilinedoes not furnish rosaniline when oxidised, but that the pre-sence of paratoluidine is essential to this reaction, this last-named substance supplying from its methyl group the methanecarbon necessary to the formation of a triphenylmethanederiv
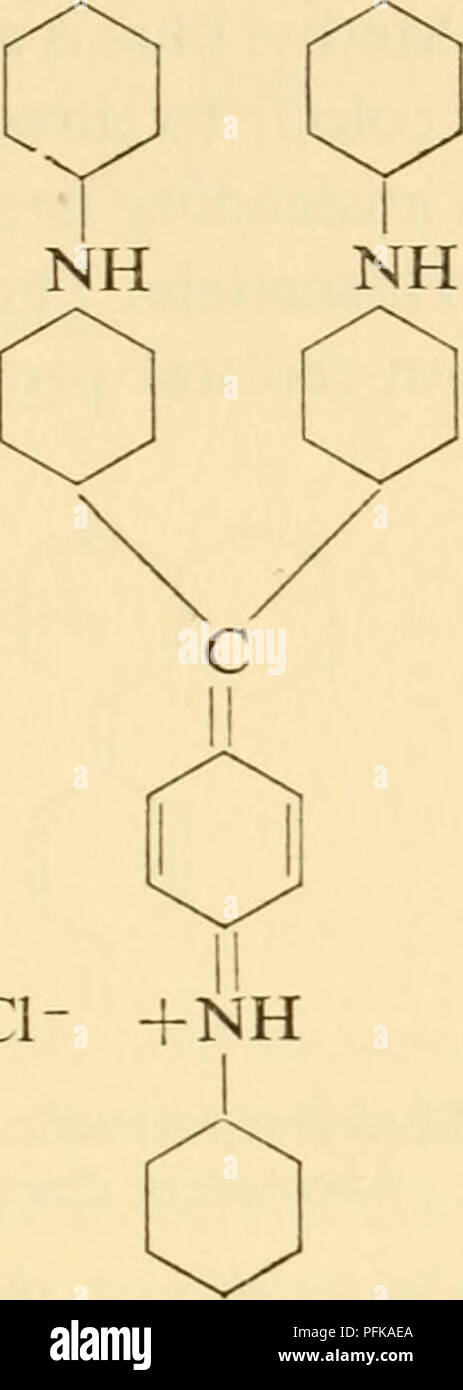 . Cytological technique; the principles underlying routine methods. Histology -- Technique; Cytology -- Technique. ci- Leitco-para- rosanil'me Skeleton-formula for triarylmethane dyes Triphenylpara- rosaniline all the many dyes that have three such rings held together in the + same way, whether any of the rings has an = NH2 group on it or not, are therefore classified together as triarylmethane dyes. In the skeleton-formula for this and other groups of dyes, the auxo- chromes and modifiers are omitted, since these differ from dye to dye. Methyl violet is pararosaniline in which four or five of Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/cytological-technique-the-principles-underlying-routine-methods-histology-technique-cytology-technique-ci-leitco-para-rosanilme-skeleton-formula-for-triarylmethane-dyes-triphenylpara-rosaniline-all-the-many-dyes-that-have-three-such-rings-held-together-in-the-same-way-whether-any-of-the-rings-has-an-=-nh2-group-on-it-or-not-are-therefore-classified-together-as-triarylmethane-dyes-in-the-skeleton-formula-for-this-and-other-groups-of-dyes-the-auxo-chromes-and-modifiers-are-omitted-since-these-differ-from-dye-to-dye-methyl-violet-is-pararosaniline-in-which-four-or-five-of-image216169586.html
. Cytological technique; the principles underlying routine methods. Histology -- Technique; Cytology -- Technique. ci- Leitco-para- rosanil'me Skeleton-formula for triarylmethane dyes Triphenylpara- rosaniline all the many dyes that have three such rings held together in the + same way, whether any of the rings has an = NH2 group on it or not, are therefore classified together as triarylmethane dyes. In the skeleton-formula for this and other groups of dyes, the auxo- chromes and modifiers are omitted, since these differ from dye to dye. Methyl violet is pararosaniline in which four or five of Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/cytological-technique-the-principles-underlying-routine-methods-histology-technique-cytology-technique-ci-leitco-para-rosanilme-skeleton-formula-for-triarylmethane-dyes-triphenylpara-rosaniline-all-the-many-dyes-that-have-three-such-rings-held-together-in-the-same-way-whether-any-of-the-rings-has-an-=-nh2-group-on-it-or-not-are-therefore-classified-together-as-triarylmethane-dyes-in-the-skeleton-formula-for-this-and-other-groups-of-dyes-the-auxo-chromes-and-modifiers-are-omitted-since-these-differ-from-dye-to-dye-methyl-violet-is-pararosaniline-in-which-four-or-five-of-image216169586.htmlRMPFKAEA–. Cytological technique; the principles underlying routine methods. Histology -- Technique; Cytology -- Technique. ci- Leitco-para- rosanil'me Skeleton-formula for triarylmethane dyes Triphenylpara- rosaniline all the many dyes that have three such rings held together in the + same way, whether any of the rings has an = NH2 group on it or not, are therefore classified together as triarylmethane dyes. In the skeleton-formula for this and other groups of dyes, the auxo- chromes and modifiers are omitted, since these differ from dye to dye. Methyl violet is pararosaniline in which four or five of
 . Chemistry of dye-stuffs. furnishes a blue rosaniline dye-stuff on oxidation. 144 CHEMISTRY OF DYE-STUFFS. furnish rosaniline dye-stuffs when oxidised. On the other-hand, such bases as contain only one amido group in thepara position and the other two in the ortho or metaposition, e.g.,. do not give dye-stuffs on oxidation. Rosanihnes containing none of the three amido groupsin the para position towards the methane carbon cannotbe prepared at all. Should one of the amido groups be lacking in rosaniline,the tinctorial character is not removed but only altered.The resulting substances—diamidotr Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/chemistry-of-dye-stuffs-furnishes-a-blue-rosaniline-dye-stuff-on-oxidation-144-chemistry-of-dye-stuffs-furnish-rosaniline-dye-stuffs-when-oxidised-on-the-other-hand-such-bases-as-contain-only-one-amido-group-in-thepara-position-and-the-other-two-in-the-ortho-or-metaposition-eg-do-not-give-dye-stuffs-on-oxidation-rosanihnes-containing-none-of-the-three-amido-groupsin-the-para-position-towards-the-methane-carbon-cannotbe-prepared-at-all-should-one-of-the-amido-groups-be-lacking-in-rosanilinethe-tinctorial-character-is-not-removed-but-only-alteredthe-resulting-substancesdiamidotr-image336723626.html
. Chemistry of dye-stuffs. furnishes a blue rosaniline dye-stuff on oxidation. 144 CHEMISTRY OF DYE-STUFFS. furnish rosaniline dye-stuffs when oxidised. On the other-hand, such bases as contain only one amido group in thepara position and the other two in the ortho or metaposition, e.g.,. do not give dye-stuffs on oxidation. Rosanihnes containing none of the three amido groupsin the para position towards the methane carbon cannotbe prepared at all. Should one of the amido groups be lacking in rosaniline,the tinctorial character is not removed but only altered.The resulting substances—diamidotr Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/chemistry-of-dye-stuffs-furnishes-a-blue-rosaniline-dye-stuff-on-oxidation-144-chemistry-of-dye-stuffs-furnish-rosaniline-dye-stuffs-when-oxidised-on-the-other-hand-such-bases-as-contain-only-one-amido-group-in-thepara-position-and-the-other-two-in-the-ortho-or-metaposition-eg-do-not-give-dye-stuffs-on-oxidation-rosanihnes-containing-none-of-the-three-amido-groupsin-the-para-position-towards-the-methane-carbon-cannotbe-prepared-at-all-should-one-of-the-amido-groups-be-lacking-in-rosanilinethe-tinctorial-character-is-not-removed-but-only-alteredthe-resulting-substancesdiamidotr-image336723626.htmlRM2AFR2BP–. Chemistry of dye-stuffs. furnishes a blue rosaniline dye-stuff on oxidation. 144 CHEMISTRY OF DYE-STUFFS. furnish rosaniline dye-stuffs when oxidised. On the other-hand, such bases as contain only one amido group in thepara position and the other two in the ortho or metaposition, e.g.,. do not give dye-stuffs on oxidation. Rosanihnes containing none of the three amido groupsin the para position towards the methane carbon cannotbe prepared at all. Should one of the amido groups be lacking in rosaniline,the tinctorial character is not removed but only altered.The resulting substances—diamidotr
 . Cytological technique; the principles underlying routine methods. Histology -- Technique; Cytology -- Technique. THE CHEMICAL COMPOSITION OF DYES 91 Beyond auxochromes and chromophores, dyes often possess atoms or groups of atoms that are called modifiers. Thus rosani- NH NH,. + Rosaniline line differs from pararosaniline only in the possession of a single methyl group; this modifies the colour, making it very slightly bluer. (Basic fuchsine is a mixture of these two closely related dyes.) The methyl group is here a modifier. It has been mentioned that the hydrogens of basic auxochromes may Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/cytological-technique-the-principles-underlying-routine-methods-histology-technique-cytology-technique-the-chemical-composition-of-dyes-91-beyond-auxochromes-and-chromophores-dyes-often-possess-atoms-or-groups-of-atoms-that-are-called-modifiers-thus-rosani-nh-nh-rosaniline-line-differs-from-pararosaniline-only-in-the-possession-of-a-single-methyl-group-this-modifies-the-colour-making-it-very-slightly-bluer-basic-fuchsine-is-a-mixture-of-these-two-closely-related-dyes-the-methyl-group-is-here-a-modifier-it-has-been-mentioned-that-the-hydrogens-of-basic-auxochromes-may-image216169608.html
. Cytological technique; the principles underlying routine methods. Histology -- Technique; Cytology -- Technique. THE CHEMICAL COMPOSITION OF DYES 91 Beyond auxochromes and chromophores, dyes often possess atoms or groups of atoms that are called modifiers. Thus rosani- NH NH,. + Rosaniline line differs from pararosaniline only in the possession of a single methyl group; this modifies the colour, making it very slightly bluer. (Basic fuchsine is a mixture of these two closely related dyes.) The methyl group is here a modifier. It has been mentioned that the hydrogens of basic auxochromes may Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/cytological-technique-the-principles-underlying-routine-methods-histology-technique-cytology-technique-the-chemical-composition-of-dyes-91-beyond-auxochromes-and-chromophores-dyes-often-possess-atoms-or-groups-of-atoms-that-are-called-modifiers-thus-rosani-nh-nh-rosaniline-line-differs-from-pararosaniline-only-in-the-possession-of-a-single-methyl-group-this-modifies-the-colour-making-it-very-slightly-bluer-basic-fuchsine-is-a-mixture-of-these-two-closely-related-dyes-the-methyl-group-is-here-a-modifier-it-has-been-mentioned-that-the-hydrogens-of-basic-auxochromes-may-image216169608.htmlRMPFKAF4–. Cytological technique; the principles underlying routine methods. Histology -- Technique; Cytology -- Technique. THE CHEMICAL COMPOSITION OF DYES 91 Beyond auxochromes and chromophores, dyes often possess atoms or groups of atoms that are called modifiers. Thus rosani- NH NH,. + Rosaniline line differs from pararosaniline only in the possession of a single methyl group; this modifies the colour, making it very slightly bluer. (Basic fuchsine is a mixture of these two closely related dyes.) The methyl group is here a modifier. It has been mentioned that the hydrogens of basic auxochromes may
![Larousse universel en 2 volumes; nouveau dictionnaire encyclopédique publié sous la direction de Claude Augé . palis-sandre. N. f. Erpét. Espèce de couleuvre. — Encycl. Les principaux colorants violets em-ployés jadis en teinture étaient : la pourpre du mu-rex, la cochenille ammoniacale. Vorseille, les boisrouges et la garance sur mordants de fer ; aujour-dhui, on utilise les couleurs artificielles dérivant dugroupe de 1 alizarine ou de la rosaniline violetHoffmann, violet de Paris). Parmi les pigmentsminéraux, citons : les laques, Voutremer < ? ] ?verres se colorent en violet en présence d Stock Photo Larousse universel en 2 volumes; nouveau dictionnaire encyclopédique publié sous la direction de Claude Augé . palis-sandre. N. f. Erpét. Espèce de couleuvre. — Encycl. Les principaux colorants violets em-ployés jadis en teinture étaient : la pourpre du mu-rex, la cochenille ammoniacale. Vorseille, les boisrouges et la garance sur mordants de fer ; aujour-dhui, on utilise les couleurs artificielles dérivant dugroupe de 1 alizarine ou de la rosaniline violetHoffmann, violet de Paris). Parmi les pigmentsminéraux, citons : les laques, Voutremer < ? ] ?verres se colorent en violet en présence d Stock Photo](https://c8.alamy.com/comp/2AKGGNE/larousse-universel-en-2-volumes-nouveau-dictionnaire-encyclopdique-publi-sous-la-direction-de-claude-aug-palis-sandre-n-f-erpt-espce-de-couleuvre-encycl-les-principaux-colorants-violets-em-ploys-jadis-en-teinture-taient-la-pourpre-du-mu-rex-la-cochenille-ammoniacale-vorseille-les-boisrouges-et-la-garance-sur-mordants-de-fer-aujour-dhui-on-utilise-les-couleurs-artificielles-drivant-dugroupe-de-1-alizarine-ou-de-la-rosaniline-violethoffmann-violet-de-paris-parmi-les-pigmentsminraux-citons-les-laques-voutremer-lt-verres-se-colorent-en-violet-en-prsence-d-2AKGGNE.jpg) Larousse universel en 2 volumes; nouveau dictionnaire encyclopédique publié sous la direction de Claude Augé . palis-sandre. N. f. Erpét. Espèce de couleuvre. — Encycl. Les principaux colorants violets em-ployés jadis en teinture étaient : la pourpre du mu-rex, la cochenille ammoniacale. Vorseille, les boisrouges et la garance sur mordants de fer ; aujour-dhui, on utilise les couleurs artificielles dérivant dugroupe de 1 alizarine ou de la rosaniline violetHoffmann, violet de Paris). Parmi les pigmentsminéraux, citons : les laques, Voutremer < ? ] ?verres se colorent en violet en présence d Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/larousse-universel-en-2-volumes-nouveau-dictionnaire-encyclopdique-publi-sous-la-direction-de-claude-aug-palis-sandre-n-f-erpt-espce-de-couleuvre-encycl-les-principaux-colorants-violets-em-ploys-jadis-en-teinture-taient-la-pourpre-du-mu-rex-la-cochenille-ammoniacale-vorseille-les-boisrouges-et-la-garance-sur-mordants-de-fer-aujour-dhui-on-utilise-les-couleurs-artificielles-drivant-dugroupe-de-1-alizarine-ou-de-la-rosaniline-violethoffmann-violet-de-paris-parmi-les-pigmentsminraux-citons-les-laques-voutremer-lt-verres-se-colorent-en-violet-en-prsence-d-image339039834.html
Larousse universel en 2 volumes; nouveau dictionnaire encyclopédique publié sous la direction de Claude Augé . palis-sandre. N. f. Erpét. Espèce de couleuvre. — Encycl. Les principaux colorants violets em-ployés jadis en teinture étaient : la pourpre du mu-rex, la cochenille ammoniacale. Vorseille, les boisrouges et la garance sur mordants de fer ; aujour-dhui, on utilise les couleurs artificielles dérivant dugroupe de 1 alizarine ou de la rosaniline violetHoffmann, violet de Paris). Parmi les pigmentsminéraux, citons : les laques, Voutremer < ? ] ?verres se colorent en violet en présence d Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/larousse-universel-en-2-volumes-nouveau-dictionnaire-encyclopdique-publi-sous-la-direction-de-claude-aug-palis-sandre-n-f-erpt-espce-de-couleuvre-encycl-les-principaux-colorants-violets-em-ploys-jadis-en-teinture-taient-la-pourpre-du-mu-rex-la-cochenille-ammoniacale-vorseille-les-boisrouges-et-la-garance-sur-mordants-de-fer-aujour-dhui-on-utilise-les-couleurs-artificielles-drivant-dugroupe-de-1-alizarine-ou-de-la-rosaniline-violethoffmann-violet-de-paris-parmi-les-pigmentsminraux-citons-les-laques-voutremer-lt-verres-se-colorent-en-violet-en-prsence-d-image339039834.htmlRM2AKGGNE–Larousse universel en 2 volumes; nouveau dictionnaire encyclopédique publié sous la direction de Claude Augé . palis-sandre. N. f. Erpét. Espèce de couleuvre. — Encycl. Les principaux colorants violets em-ployés jadis en teinture étaient : la pourpre du mu-rex, la cochenille ammoniacale. Vorseille, les boisrouges et la garance sur mordants de fer ; aujour-dhui, on utilise les couleurs artificielles dérivant dugroupe de 1 alizarine ou de la rosaniline violetHoffmann, violet de Paris). Parmi les pigmentsminéraux, citons : les laques, Voutremer < ? ] ?verres se colorent en violet en présence d
 . Eighth International congress of applied chemistry : Washington and New York, September 4 to 13, 1912 ... -- . The hydrol and phosgene syntheses of triphenylmethanedyestuffs may also be regarded in a similar light. Thus theextraordinary facility with which tetramethyldiamidobenzhydrolundergoes condensation with a wide range of substances isexplained if we regard these reactions as due to the additivecapacity of the hydrol salt in its quinonoid form: 466 Original Communications: Eighth International [vol. Even the phenylation of rosaniline in the production ofAniline Blue may be regarded with Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/eighth-international-congress-of-applied-chemistry-washington-and-new-york-september-4-to-13-1912-the-hydrol-and-phosgene-syntheses-of-triphenylmethanedyestuffs-may-also-be-regarded-in-a-similar-light-thus-theextraordinary-facility-with-which-tetramethyldiamidobenzhydrolundergoes-condensation-with-a-wide-range-of-substances-isexplained-if-we-regard-these-reactions-as-due-to-the-additivecapacity-of-the-hydrol-salt-in-its-quinonoid-form-466-original-communications-eighth-international-vol-even-the-phenylation-of-rosaniline-in-the-production-ofaniline-blue-may-be-regarded-with-image372283442.html
. Eighth International congress of applied chemistry : Washington and New York, September 4 to 13, 1912 ... -- . The hydrol and phosgene syntheses of triphenylmethanedyestuffs may also be regarded in a similar light. Thus theextraordinary facility with which tetramethyldiamidobenzhydrolundergoes condensation with a wide range of substances isexplained if we regard these reactions as due to the additivecapacity of the hydrol salt in its quinonoid form: 466 Original Communications: Eighth International [vol. Even the phenylation of rosaniline in the production ofAniline Blue may be regarded with Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/eighth-international-congress-of-applied-chemistry-washington-and-new-york-september-4-to-13-1912-the-hydrol-and-phosgene-syntheses-of-triphenylmethanedyestuffs-may-also-be-regarded-in-a-similar-light-thus-theextraordinary-facility-with-which-tetramethyldiamidobenzhydrolundergoes-condensation-with-a-wide-range-of-substances-isexplained-if-we-regard-these-reactions-as-due-to-the-additivecapacity-of-the-hydrol-salt-in-its-quinonoid-form-466-original-communications-eighth-international-vol-even-the-phenylation-of-rosaniline-in-the-production-ofaniline-blue-may-be-regarded-with-image372283442.htmlRM2CHJY96–. Eighth International congress of applied chemistry : Washington and New York, September 4 to 13, 1912 ... -- . The hydrol and phosgene syntheses of triphenylmethanedyestuffs may also be regarded in a similar light. Thus theextraordinary facility with which tetramethyldiamidobenzhydrolundergoes condensation with a wide range of substances isexplained if we regard these reactions as due to the additivecapacity of the hydrol salt in its quinonoid form: 466 Original Communications: Eighth International [vol. Even the phenylation of rosaniline in the production ofAniline Blue may be regarded with
 . A practical handbook of dyeing and calico-printing. With eleven page-plates, forty-seven specimens of dyed and printed fabrics, and thirty-eight woodcuts . aphthylamin or with bromated naphthalin, gives a fine violet. 26. Naphthoic Blue.—The attempts made by Girard, and by Mylius, ofDasle, to replace benzoic acid with naphthoic acid in the conversion ofmagenta into rosaniline blue, have shown that the latter, which is cheaper,gives a blue at least as bright and beautiful as that produced by means ofbenzoic acid. 27.— Aniline Grey (Castelhaz).— Aniline grey (Gris Castelhaz, p. 210) 1 gallon. Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/a-practical-handbook-of-dyeing-and-calico-printing-with-eleven-page-plates-forty-seven-specimens-of-dyed-and-printed-fabrics-and-thirty-eight-woodcuts-aphthylamin-or-with-bromated-naphthalin-gives-a-fine-violet-26-naphthoic-bluethe-attempts-made-by-girard-and-by-mylius-ofdasle-to-replace-benzoic-acid-with-naphthoic-acid-in-the-conversion-ofmagenta-into-rosaniline-blue-have-shown-that-the-latter-which-is-cheapergives-a-blue-at-least-as-bright-and-beautiful-as-that-produced-by-means-ofbenzoic-acid-27-aniline-grey-castelhaz-aniline-grey-gris-castelhaz-p-210-1-gallon-image370064516.html
. A practical handbook of dyeing and calico-printing. With eleven page-plates, forty-seven specimens of dyed and printed fabrics, and thirty-eight woodcuts . aphthylamin or with bromated naphthalin, gives a fine violet. 26. Naphthoic Blue.—The attempts made by Girard, and by Mylius, ofDasle, to replace benzoic acid with naphthoic acid in the conversion ofmagenta into rosaniline blue, have shown that the latter, which is cheaper,gives a blue at least as bright and beautiful as that produced by means ofbenzoic acid. 27.— Aniline Grey (Castelhaz).— Aniline grey (Gris Castelhaz, p. 210) 1 gallon. Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/a-practical-handbook-of-dyeing-and-calico-printing-with-eleven-page-plates-forty-seven-specimens-of-dyed-and-printed-fabrics-and-thirty-eight-woodcuts-aphthylamin-or-with-bromated-naphthalin-gives-a-fine-violet-26-naphthoic-bluethe-attempts-made-by-girard-and-by-mylius-ofdasle-to-replace-benzoic-acid-with-naphthoic-acid-in-the-conversion-ofmagenta-into-rosaniline-blue-have-shown-that-the-latter-which-is-cheapergives-a-blue-at-least-as-bright-and-beautiful-as-that-produced-by-means-ofbenzoic-acid-27-aniline-grey-castelhaz-aniline-grey-gris-castelhaz-p-210-1-gallon-image370064516.htmlRM2CE1W1T–. A practical handbook of dyeing and calico-printing. With eleven page-plates, forty-seven specimens of dyed and printed fabrics, and thirty-eight woodcuts . aphthylamin or with bromated naphthalin, gives a fine violet. 26. Naphthoic Blue.—The attempts made by Girard, and by Mylius, ofDasle, to replace benzoic acid with naphthoic acid in the conversion ofmagenta into rosaniline blue, have shown that the latter, which is cheaper,gives a blue at least as bright and beautiful as that produced by means ofbenzoic acid. 27.— Aniline Grey (Castelhaz).— Aniline grey (Gris Castelhaz, p. 210) 1 gallon.
 . Official and provisional methods of analysis. low, Ponceau, Bor-deaux, and Congo red. Class II. Indogenide and imido-quinone coloringmatters, methyleneblue, safranin, indi-go-carmine. Decolorization or a pre-cipitate. Imido -car-bo-quinone coloringmatters. Class III. Amido - derivatives ofdi and triphenyl-me-tnane, auramines,acridines, quinolines,and color derivativesof thio benzenil. Fuchsin, rosaniline, au-ramine. No precipitation. Liq-uid becomes more col-ored. Oxy-carbo-qui-none coloring matters. Class rv. Nonamide dip hen yl-methane. oxy-ketone,and most of naturalorganic coloring mat-te Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/official-and-provisional-methods-of-analysis-low-ponceau-bor-deaux-and-congo-red-class-ii-indogenide-and-imido-quinone-coloringmatters-methyleneblue-safranin-indi-go-carmine-decolorization-or-a-pre-cipitate-imido-car-bo-quinone-coloringmatters-class-iii-amido-derivatives-ofdi-and-triphenyl-me-tnane-auraminesacridines-quinolinesand-color-derivativesof-thio-benzenil-fuchsin-rosaniline-au-ramine-no-precipitation-liq-uid-becomes-more-col-ored-oxy-carbo-qui-none-coloring-matters-class-rv-nonamide-dip-hen-yl-methane-oxy-ketoneand-most-of-naturalorganic-coloring-mat-te-image370473986.html
. Official and provisional methods of analysis. low, Ponceau, Bor-deaux, and Congo red. Class II. Indogenide and imido-quinone coloringmatters, methyleneblue, safranin, indi-go-carmine. Decolorization or a pre-cipitate. Imido -car-bo-quinone coloringmatters. Class III. Amido - derivatives ofdi and triphenyl-me-tnane, auramines,acridines, quinolines,and color derivativesof thio benzenil. Fuchsin, rosaniline, au-ramine. No precipitation. Liq-uid becomes more col-ored. Oxy-carbo-qui-none coloring matters. Class rv. Nonamide dip hen yl-methane. oxy-ketone,and most of naturalorganic coloring mat-te Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/official-and-provisional-methods-of-analysis-low-ponceau-bor-deaux-and-congo-red-class-ii-indogenide-and-imido-quinone-coloringmatters-methyleneblue-safranin-indi-go-carmine-decolorization-or-a-pre-cipitate-imido-car-bo-quinone-coloringmatters-class-iii-amido-derivatives-ofdi-and-triphenyl-me-tnane-auraminesacridines-quinolinesand-color-derivativesof-thio-benzenil-fuchsin-rosaniline-au-ramine-no-precipitation-liq-uid-becomes-more-col-ored-oxy-carbo-qui-none-coloring-matters-class-rv-nonamide-dip-hen-yl-methane-oxy-ketoneand-most-of-naturalorganic-coloring-mat-te-image370473986.htmlRM2CEMF9P–. Official and provisional methods of analysis. low, Ponceau, Bor-deaux, and Congo red. Class II. Indogenide and imido-quinone coloringmatters, methyleneblue, safranin, indi-go-carmine. Decolorization or a pre-cipitate. Imido -car-bo-quinone coloringmatters. Class III. Amido - derivatives ofdi and triphenyl-me-tnane, auramines,acridines, quinolines,and color derivativesof thio benzenil. Fuchsin, rosaniline, au-ramine. No precipitation. Liq-uid becomes more col-ored. Oxy-carbo-qui-none coloring matters. Class rv. Nonamide dip hen yl-methane. oxy-ketone,and most of naturalorganic coloring mat-te
 . A practical handbook of dyeing and calico-printing. With eleven page-plates, forty-seven specimens of dyed and printed fabrics, and thirty-eight woodcuts . covered. Of the Hofmann violets, chiefly manufactured by Messrs. Brooke, Simpson,and Spiller, there are a variety of shades, ranging from the reddest (R.R.R.) tothe bluest (B.B.B.). These marks correspond to certain different gradesof substitution, ethyl or methyl here taking the place of hydrogen, in therosaniline atom for atom. The shade R.R.R. consists chiefly of mono-methylated rosaniline, whilst B B B, is almost exclusively composed Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/a-practical-handbook-of-dyeing-and-calico-printing-with-eleven-page-plates-forty-seven-specimens-of-dyed-and-printed-fabrics-and-thirty-eight-woodcuts-covered-of-the-hofmann-violets-chiefly-manufactured-by-messrs-brooke-simpsonand-spiller-there-are-a-variety-of-shades-ranging-from-the-reddest-rrr-tothe-bluest-bbb-these-marks-correspond-to-certain-different-gradesof-substitution-ethyl-or-methyl-here-taking-the-place-of-hydrogen-in-therosaniline-atom-for-atom-the-shade-rrr-consists-chiefly-of-mono-methylated-rosaniline-whilst-b-b-b-is-almost-exclusively-composed-image370066779.html
. A practical handbook of dyeing and calico-printing. With eleven page-plates, forty-seven specimens of dyed and printed fabrics, and thirty-eight woodcuts . covered. Of the Hofmann violets, chiefly manufactured by Messrs. Brooke, Simpson,and Spiller, there are a variety of shades, ranging from the reddest (R.R.R.) tothe bluest (B.B.B.). These marks correspond to certain different gradesof substitution, ethyl or methyl here taking the place of hydrogen, in therosaniline atom for atom. The shade R.R.R. consists chiefly of mono-methylated rosaniline, whilst B B B, is almost exclusively composed Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/a-practical-handbook-of-dyeing-and-calico-printing-with-eleven-page-plates-forty-seven-specimens-of-dyed-and-printed-fabrics-and-thirty-eight-woodcuts-covered-of-the-hofmann-violets-chiefly-manufactured-by-messrs-brooke-simpsonand-spiller-there-are-a-variety-of-shades-ranging-from-the-reddest-rrr-tothe-bluest-bbb-these-marks-correspond-to-certain-different-gradesof-substitution-ethyl-or-methyl-here-taking-the-place-of-hydrogen-in-therosaniline-atom-for-atom-the-shade-rrr-consists-chiefly-of-mono-methylated-rosaniline-whilst-b-b-b-is-almost-exclusively-composed-image370066779.htmlRM2CE1YXK–. A practical handbook of dyeing and calico-printing. With eleven page-plates, forty-seven specimens of dyed and printed fabrics, and thirty-eight woodcuts . covered. Of the Hofmann violets, chiefly manufactured by Messrs. Brooke, Simpson,and Spiller, there are a variety of shades, ranging from the reddest (R.R.R.) tothe bluest (B.B.B.). These marks correspond to certain different gradesof substitution, ethyl or methyl here taking the place of hydrogen, in therosaniline atom for atom. The shade R.R.R. consists chiefly of mono-methylated rosaniline, whilst B B B, is almost exclusively composed
 . Archives des sciences physiques et naturelles. ion déterminées par les dosages.La courbe (supposée) AB délimiterait les quantités decolorants dégorgeables et celle restant comme teinture. B fig. 2. La surface ABCD représenterait donc la teintureaux différentes concentrations1. Pour compléter ce résumé, je dois rappeler un faittrès important observé par Freundlich. Si nous consi-dérons une molécule de matière colorante basique quelon utilise constamment à létat de sel et que nouspouvons représenter par R HCl où R désigne la basecolorante complexe, rosaniline dans le cas de la fuch-sine, dimét Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/archives-des-sciences-physiques-et-naturelles-ion-dtermines-par-les-dosagesla-courbe-suppose-ab-dlimiterait-les-quantits-decolorants-dgorgeables-et-celle-restant-comme-teinture-b-fig-2-la-surface-abcd-reprsenterait-donc-la-teintureaux-diffrentes-concentrations1-pour-complter-ce-rsum-je-dois-rappeler-un-faittrs-important-observ-par-freundlich-si-nous-consi-drons-une-molcule-de-matire-colorante-basique-quelon-utilise-constamment-ltat-de-sel-et-que-nouspouvons-reprsenter-par-r-hcl-o-r-dsigne-la-basecolorante-complexe-rosaniline-dans-le-cas-de-la-fuch-sine-dimt-image370493168.html
. Archives des sciences physiques et naturelles. ion déterminées par les dosages.La courbe (supposée) AB délimiterait les quantités decolorants dégorgeables et celle restant comme teinture. B fig. 2. La surface ABCD représenterait donc la teintureaux différentes concentrations1. Pour compléter ce résumé, je dois rappeler un faittrès important observé par Freundlich. Si nous consi-dérons une molécule de matière colorante basique quelon utilise constamment à létat de sel et que nouspouvons représenter par R HCl où R désigne la basecolorante complexe, rosaniline dans le cas de la fuch-sine, dimét Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/archives-des-sciences-physiques-et-naturelles-ion-dtermines-par-les-dosagesla-courbe-suppose-ab-dlimiterait-les-quantits-decolorants-dgorgeables-et-celle-restant-comme-teinture-b-fig-2-la-surface-abcd-reprsenterait-donc-la-teintureaux-diffrentes-concentrations1-pour-complter-ce-rsum-je-dois-rappeler-un-faittrs-important-observ-par-freundlich-si-nous-consi-drons-une-molcule-de-matire-colorante-basique-quelon-utilise-constamment-ltat-de-sel-et-que-nouspouvons-reprsenter-par-r-hcl-o-r-dsigne-la-basecolorante-complexe-rosaniline-dans-le-cas-de-la-fuch-sine-dimt-image370493168.htmlRM2CENBPT–. Archives des sciences physiques et naturelles. ion déterminées par les dosages.La courbe (supposée) AB délimiterait les quantités decolorants dégorgeables et celle restant comme teinture. B fig. 2. La surface ABCD représenterait donc la teintureaux différentes concentrations1. Pour compléter ce résumé, je dois rappeler un faittrès important observé par Freundlich. Si nous consi-dérons une molécule de matière colorante basique quelon utilise constamment à létat de sel et que nouspouvons représenter par R HCl où R désigne la basecolorante complexe, rosaniline dans le cas de la fuch-sine, dimét
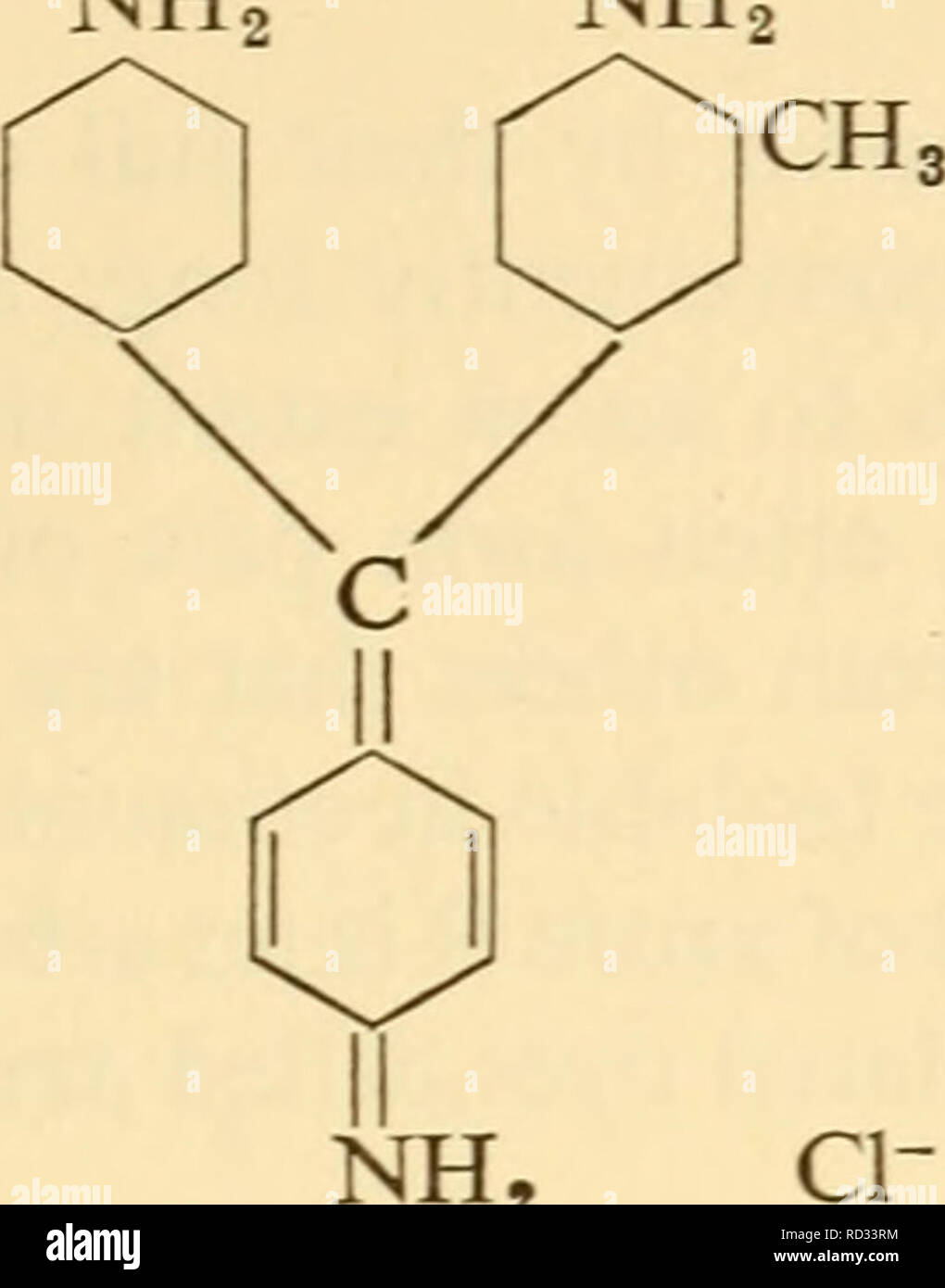 . Cytological technique; the principles underlying routine methods. Histology -- Technique; Cytology -- Technique. THE CHEMICAL COMPOSITION OF DYES 91 Beyond auxochromes and chromophores, dyes often possess atoms or groups of atoms that are called modifiers. Thus rosani- NH NH,. + Rosaniline line differs from pararosaniline only in the possession of a single methyl group; this modifies the colour, making it very slightly bluer. (Basic fuchsine is a mixture of these two closely related dyes.) The methyl group is here a modifier. It has been mentioned that the hydrogens of basic auxochromes may Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/cytological-technique-the-principles-underlying-routine-methods-histology-technique-cytology-technique-the-chemical-composition-of-dyes-91-beyond-auxochromes-and-chromophores-dyes-often-possess-atoms-or-groups-of-atoms-that-are-called-modifiers-thus-rosani-nh-nh-rosaniline-line-differs-from-pararosaniline-only-in-the-possession-of-a-single-methyl-group-this-modifies-the-colour-making-it-very-slightly-bluer-basic-fuchsine-is-a-mixture-of-these-two-closely-related-dyes-the-methyl-group-is-here-a-modifier-it-has-been-mentioned-that-the-hydrogens-of-basic-auxochromes-may-image231794184.html
. Cytological technique; the principles underlying routine methods. Histology -- Technique; Cytology -- Technique. THE CHEMICAL COMPOSITION OF DYES 91 Beyond auxochromes and chromophores, dyes often possess atoms or groups of atoms that are called modifiers. Thus rosani- NH NH,. + Rosaniline line differs from pararosaniline only in the possession of a single methyl group; this modifies the colour, making it very slightly bluer. (Basic fuchsine is a mixture of these two closely related dyes.) The methyl group is here a modifier. It has been mentioned that the hydrogens of basic auxochromes may Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/cytological-technique-the-principles-underlying-routine-methods-histology-technique-cytology-technique-the-chemical-composition-of-dyes-91-beyond-auxochromes-and-chromophores-dyes-often-possess-atoms-or-groups-of-atoms-that-are-called-modifiers-thus-rosani-nh-nh-rosaniline-line-differs-from-pararosaniline-only-in-the-possession-of-a-single-methyl-group-this-modifies-the-colour-making-it-very-slightly-bluer-basic-fuchsine-is-a-mixture-of-these-two-closely-related-dyes-the-methyl-group-is-here-a-modifier-it-has-been-mentioned-that-the-hydrogens-of-basic-auxochromes-may-image231794184.htmlRMRD33RM–. Cytological technique; the principles underlying routine methods. Histology -- Technique; Cytology -- Technique. THE CHEMICAL COMPOSITION OF DYES 91 Beyond auxochromes and chromophores, dyes often possess atoms or groups of atoms that are called modifiers. Thus rosani- NH NH,. + Rosaniline line differs from pararosaniline only in the possession of a single methyl group; this modifies the colour, making it very slightly bluer. (Basic fuchsine is a mixture of these two closely related dyes.) The methyl group is here a modifier. It has been mentioned that the hydrogens of basic auxochromes may
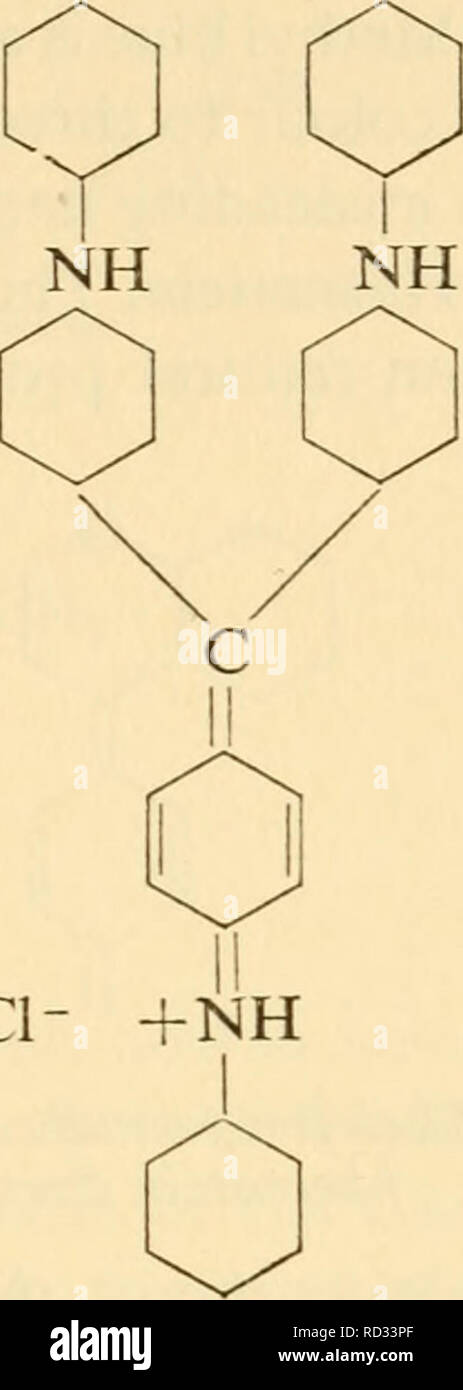 . Cytological technique; the principles underlying routine methods. Histology -- Technique; Cytology -- Technique. ci- Leitco-para- rosanil'me Skeleton-formula for triarylmethane dyes Triphenylpara- rosaniline all the many dyes that have three such rings held together in the + same way, whether any of the rings has an = NH2 group on it or not, are therefore classified together as triarylmethane dyes. In the skeleton-formula for this and other groups of dyes, the auxo- chromes and modifiers are omitted, since these differ from dye to dye. Methyl violet is pararosaniline in which four or five of Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/cytological-technique-the-principles-underlying-routine-methods-histology-technique-cytology-technique-ci-leitco-para-rosanilme-skeleton-formula-for-triarylmethane-dyes-triphenylpara-rosaniline-all-the-many-dyes-that-have-three-such-rings-held-together-in-the-same-way-whether-any-of-the-rings-has-an-=-nh2-group-on-it-or-not-are-therefore-classified-together-as-triarylmethane-dyes-in-the-skeleton-formula-for-this-and-other-groups-of-dyes-the-auxo-chromes-and-modifiers-are-omitted-since-these-differ-from-dye-to-dye-methyl-violet-is-pararosaniline-in-which-four-or-five-of-image231794151.html
. Cytological technique; the principles underlying routine methods. Histology -- Technique; Cytology -- Technique. ci- Leitco-para- rosanil'me Skeleton-formula for triarylmethane dyes Triphenylpara- rosaniline all the many dyes that have three such rings held together in the + same way, whether any of the rings has an = NH2 group on it or not, are therefore classified together as triarylmethane dyes. In the skeleton-formula for this and other groups of dyes, the auxo- chromes and modifiers are omitted, since these differ from dye to dye. Methyl violet is pararosaniline in which four or five of Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/cytological-technique-the-principles-underlying-routine-methods-histology-technique-cytology-technique-ci-leitco-para-rosanilme-skeleton-formula-for-triarylmethane-dyes-triphenylpara-rosaniline-all-the-many-dyes-that-have-three-such-rings-held-together-in-the-same-way-whether-any-of-the-rings-has-an-=-nh2-group-on-it-or-not-are-therefore-classified-together-as-triarylmethane-dyes-in-the-skeleton-formula-for-this-and-other-groups-of-dyes-the-auxo-chromes-and-modifiers-are-omitted-since-these-differ-from-dye-to-dye-methyl-violet-is-pararosaniline-in-which-four-or-five-of-image231794151.htmlRMRD33PF–. Cytological technique; the principles underlying routine methods. Histology -- Technique; Cytology -- Technique. ci- Leitco-para- rosanil'me Skeleton-formula for triarylmethane dyes Triphenylpara- rosaniline all the many dyes that have three such rings held together in the + same way, whether any of the rings has an = NH2 group on it or not, are therefore classified together as triarylmethane dyes. In the skeleton-formula for this and other groups of dyes, the auxo- chromes and modifiers are omitted, since these differ from dye to dye. Methyl violet is pararosaniline in which four or five of
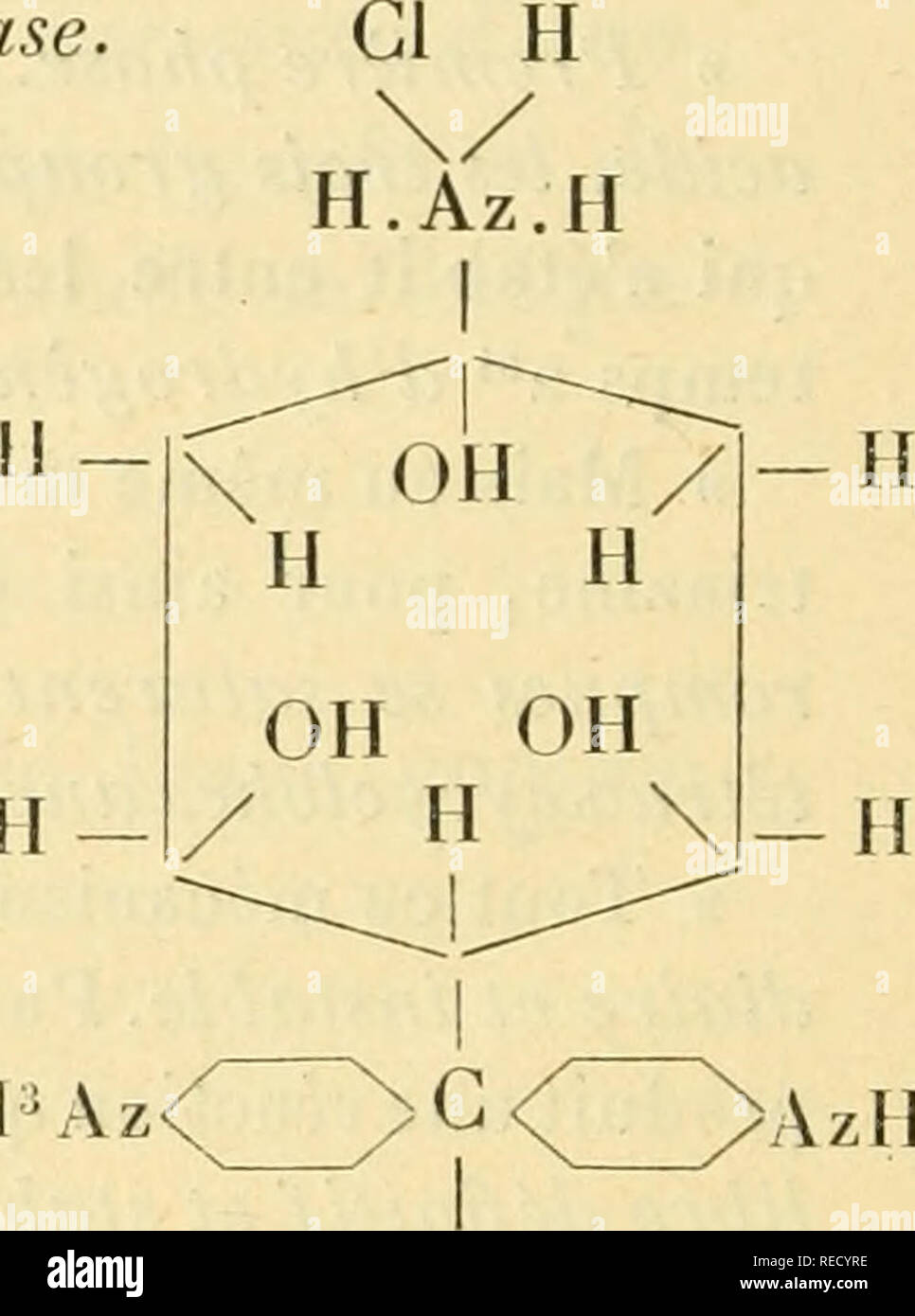 . Comptes rendus hebdomadaires des séances de l'Académie des sciences. Science -- Societies, etc; Science; Science. H* A .Monochlorhydrate ( fuchsine ) coloré. Trichlorhydrate de rosaniline coloré et instable. H â CIU^Vz. AzIPCl on Tétraoxycyclohexanerosaniline incolore et stable à l'état triacide. » 3° Dissolution du carbinol dans un excès d'acide. â On obtient ici aussi, au premier moment, le sel monoacide de tétraoxycyclohexanerosaniline qui renferme la chaîne triazine'; mais instantanément Vexcès d'acide parvient à enlever la chaîne triazine et rend le dérivé incolore de l' Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/comptes-rendus-hebdomadaires-des-sances-de-lacadmie-des-sciences-science-societies-etc-science-science-h-a-monochlorhydrate-fuchsine-color-trichlorhydrate-de-rosaniline-color-et-instable-h-ciuvz-azipcl-on-ttraoxycyclohexanerosaniline-incolore-et-stable-ltat-triacide-3-dissolution-du-carbinol-dans-un-excs-dacide-on-obtient-ici-aussi-au-premier-moment-le-sel-monoacide-de-ttraoxycyclohexanerosaniline-qui-renferme-la-chane-triazine-mais-instantanment-vexcs-dacide-parvient-enlever-la-chane-triazine-et-rend-le-driv-incolore-de-l-image232625218.html
. Comptes rendus hebdomadaires des séances de l'Académie des sciences. Science -- Societies, etc; Science; Science. H* A .Monochlorhydrate ( fuchsine ) coloré. Trichlorhydrate de rosaniline coloré et instable. H â CIU^Vz. AzIPCl on Tétraoxycyclohexanerosaniline incolore et stable à l'état triacide. » 3° Dissolution du carbinol dans un excès d'acide. â On obtient ici aussi, au premier moment, le sel monoacide de tétraoxycyclohexanerosaniline qui renferme la chaîne triazine'; mais instantanément Vexcès d'acide parvient à enlever la chaîne triazine et rend le dérivé incolore de l' Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/comptes-rendus-hebdomadaires-des-sances-de-lacadmie-des-sciences-science-societies-etc-science-science-h-a-monochlorhydrate-fuchsine-color-trichlorhydrate-de-rosaniline-color-et-instable-h-ciuvz-azipcl-on-ttraoxycyclohexanerosaniline-incolore-et-stable-ltat-triacide-3-dissolution-du-carbinol-dans-un-excs-dacide-on-obtient-ici-aussi-au-premier-moment-le-sel-monoacide-de-ttraoxycyclohexanerosaniline-qui-renferme-la-chane-triazine-mais-instantanment-vexcs-dacide-parvient-enlever-la-chane-triazine-et-rend-le-driv-incolore-de-l-image232625218.htmlRMRECYRE–. Comptes rendus hebdomadaires des séances de l'Académie des sciences. Science -- Societies, etc; Science; Science. H* A .Monochlorhydrate ( fuchsine ) coloré. Trichlorhydrate de rosaniline coloré et instable. H â CIU^Vz. AzIPCl on Tétraoxycyclohexanerosaniline incolore et stable à l'état triacide. » 3° Dissolution du carbinol dans un excès d'acide. â On obtient ici aussi, au premier moment, le sel monoacide de tétraoxycyclohexanerosaniline qui renferme la chaîne triazine'; mais instantanément Vexcès d'acide parvient à enlever la chaîne triazine et rend le dérivé incolore de l'
 . comptesrendusheb1391904acad. es naturelles. H^Az Monochlorhydrale ( fuchsine ) colore. Deuxième phase. II- H - Cl H H.Az.H Trichlorhydratc de rosaniline coloré cl instable. ClH'Az. II AzFPCl nll Télraoxycycloliexanerosaniline incolore et stable à l'état triacide. » 3° Dissolution du carbinol dans un excès d'acide. â On obtient ici aussi, au premier moment, le sel monoacide de tétraoxycyclohexanerosanilinc qui renferme la chaîne triazine; mais instantanément l'excès d'acide parvient à enlever la chaîne triazine et rend le dérivé incolore de l'hcxahydrobenzine stable comme sel tr Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/comptesrendusheb1391904acad-es-naturelles-haz-monochlorhydrale-fuchsine-colore-deuxime-phase-ii-h-cl-h-hazh-trichlorhydratc-de-rosaniline-color-cl-instable-clhaz-ii-azfpcl-nll-tlraoxycycloliexanerosaniline-incolore-et-stable-ltat-triacide-3-dissolution-du-carbinol-dans-un-excs-dacide-on-obtient-ici-aussi-au-premier-moment-le-sel-monoacide-de-ttraoxycyclohexanerosanilinc-qui-renferme-la-chane-triazine-mais-instantanment-lexcs-dacide-parvient-enlever-la-chane-triazine-et-rend-le-driv-incolore-de-lhcxahydrobenzine-stable-comme-sel-tr-image232622204.html
. comptesrendusheb1391904acad. es naturelles. H^Az Monochlorhydrale ( fuchsine ) colore. Deuxième phase. II- H - Cl H H.Az.H Trichlorhydratc de rosaniline coloré cl instable. ClH'Az. II AzFPCl nll Télraoxycycloliexanerosaniline incolore et stable à l'état triacide. » 3° Dissolution du carbinol dans un excès d'acide. â On obtient ici aussi, au premier moment, le sel monoacide de tétraoxycyclohexanerosanilinc qui renferme la chaîne triazine; mais instantanément l'excès d'acide parvient à enlever la chaîne triazine et rend le dérivé incolore de l'hcxahydrobenzine stable comme sel tr Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/comptesrendusheb1391904acad-es-naturelles-haz-monochlorhydrale-fuchsine-colore-deuxime-phase-ii-h-cl-h-hazh-trichlorhydratc-de-rosaniline-color-cl-instable-clhaz-ii-azfpcl-nll-tlraoxycycloliexanerosaniline-incolore-et-stable-ltat-triacide-3-dissolution-du-carbinol-dans-un-excs-dacide-on-obtient-ici-aussi-au-premier-moment-le-sel-monoacide-de-ttraoxycyclohexanerosanilinc-qui-renferme-la-chane-triazine-mais-instantanment-lexcs-dacide-parvient-enlever-la-chane-triazine-et-rend-le-driv-incolore-de-lhcxahydrobenzine-stable-comme-sel-tr-image232622204.htmlRMRECRYT–. comptesrendusheb1391904acad. es naturelles. H^Az Monochlorhydrale ( fuchsine ) colore. Deuxième phase. II- H - Cl H H.Az.H Trichlorhydratc de rosaniline coloré cl instable. ClH'Az. II AzFPCl nll Télraoxycycloliexanerosaniline incolore et stable à l'état triacide. » 3° Dissolution du carbinol dans un excès d'acide. â On obtient ici aussi, au premier moment, le sel monoacide de tétraoxycyclohexanerosanilinc qui renferme la chaîne triazine; mais instantanément l'excès d'acide parvient à enlever la chaîne triazine et rend le dérivé incolore de l'hcxahydrobenzine stable comme sel tr
 . comptesrendusheb1391904acad. es naturelles. A.ZII- Rosanilinecarbinol au moment du contact avec un acide.. zlP Chlorhydrale de rosaniline (fuchsine) coloré et stable à l'élat monoacide. Tclraoxycyclohexanerosaniline, incolore et instable, comme sel nionoacide.. Please note that these images are extracted from scanned page images that may have been digitally enhanced for readability - coloration and appearance of these illustrations may not perfectly resemble the original work.. nce Periodicals; dicals. Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/comptesrendusheb1391904acad-es-naturelles-azii-rosanilinecarbinol-au-moment-du-contact-avec-un-acide-zlp-chlorhydrale-de-rosaniline-fuchsine-color-et-stable-llat-monoacide-tclraoxycyclohexanerosaniline-incolore-et-instable-comme-sel-nionoacide-please-note-that-these-images-are-extracted-from-scanned-page-images-that-may-have-been-digitally-enhanced-for-readability-coloration-and-appearance-of-these-illustrations-may-not-perfectly-resemble-the-original-work-nce-periodicals-dicals-image232622212.html
. comptesrendusheb1391904acad. es naturelles. A.ZII- Rosanilinecarbinol au moment du contact avec un acide.. zlP Chlorhydrale de rosaniline (fuchsine) coloré et stable à l'élat monoacide. Tclraoxycyclohexanerosaniline, incolore et instable, comme sel nionoacide.. Please note that these images are extracted from scanned page images that may have been digitally enhanced for readability - coloration and appearance of these illustrations may not perfectly resemble the original work.. nce Periodicals; dicals. Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/comptesrendusheb1391904acad-es-naturelles-azii-rosanilinecarbinol-au-moment-du-contact-avec-un-acide-zlp-chlorhydrale-de-rosaniline-fuchsine-color-et-stable-llat-monoacide-tclraoxycyclohexanerosaniline-incolore-et-instable-comme-sel-nionoacide-please-note-that-these-images-are-extracted-from-scanned-page-images-that-may-have-been-digitally-enhanced-for-readability-coloration-and-appearance-of-these-illustrations-may-not-perfectly-resemble-the-original-work-nce-periodicals-dicals-image232622212.htmlRMRECT04–. comptesrendusheb1391904acad. es naturelles. A.ZII- Rosanilinecarbinol au moment du contact avec un acide.. zlP Chlorhydrale de rosaniline (fuchsine) coloré et stable à l'élat monoacide. Tclraoxycyclohexanerosaniline, incolore et instable, comme sel nionoacide.. Please note that these images are extracted from scanned page images that may have been digitally enhanced for readability - coloration and appearance of these illustrations may not perfectly resemble the original work.. nce Periodicals; dicals.
 . Comptes rendus hebdomadaires des séances de l'Académie des sciences. Science -- Societies, etc; Science; Science. n^z. >.AzH3 Chlorhydrale de rosaniline (fuclisine) coloré et stable à l'élat monoacide. H* A Tétraoxycyclohexanerosaniline, incolore et instable, comme sel monoacide.. Please note that these images are extracted from scanned page images that may have been digitally enhanced for readability - coloration and appearance of these illustrations may not perfectly resemble the original work.. Académie des sciences (France); Centre national de la recherche scientifique (France) Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/comptes-rendus-hebdomadaires-des-sances-de-lacadmie-des-sciences-science-societies-etc-science-science-nz-gtazh3-chlorhydrale-de-rosaniline-fuclisine-color-et-stable-llat-monoacide-h-a-ttraoxycyclohexanerosaniline-incolore-et-instable-comme-sel-monoacide-please-note-that-these-images-are-extracted-from-scanned-page-images-that-may-have-been-digitally-enhanced-for-readability-coloration-and-appearance-of-these-illustrations-may-not-perfectly-resemble-the-original-work-acadmie-des-sciences-france-centre-national-de-la-recherche-scientifique-france-image232625220.html
. Comptes rendus hebdomadaires des séances de l'Académie des sciences. Science -- Societies, etc; Science; Science. n^z. >.AzH3 Chlorhydrale de rosaniline (fuclisine) coloré et stable à l'élat monoacide. H* A Tétraoxycyclohexanerosaniline, incolore et instable, comme sel monoacide.. Please note that these images are extracted from scanned page images that may have been digitally enhanced for readability - coloration and appearance of these illustrations may not perfectly resemble the original work.. Académie des sciences (France); Centre national de la recherche scientifique (France) Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/comptes-rendus-hebdomadaires-des-sances-de-lacadmie-des-sciences-science-societies-etc-science-science-nz-gtazh3-chlorhydrale-de-rosaniline-fuclisine-color-et-stable-llat-monoacide-h-a-ttraoxycyclohexanerosaniline-incolore-et-instable-comme-sel-monoacide-please-note-that-these-images-are-extracted-from-scanned-page-images-that-may-have-been-digitally-enhanced-for-readability-coloration-and-appearance-of-these-illustrations-may-not-perfectly-resemble-the-original-work-acadmie-des-sciences-france-centre-national-de-la-recherche-scientifique-france-image232625220.htmlRMRECYRG–. Comptes rendus hebdomadaires des séances de l'Académie des sciences. Science -- Societies, etc; Science; Science. n^z. >.AzH3 Chlorhydrale de rosaniline (fuclisine) coloré et stable à l'élat monoacide. H* A Tétraoxycyclohexanerosaniline, incolore et instable, comme sel monoacide.. Please note that these images are extracted from scanned page images that may have been digitally enhanced for readability - coloration and appearance of these illustrations may not perfectly resemble the original work.. Académie des sciences (France); Centre national de la recherche scientifique (France)