Quick filters:
Sal ammoniac Stock Photos and Images
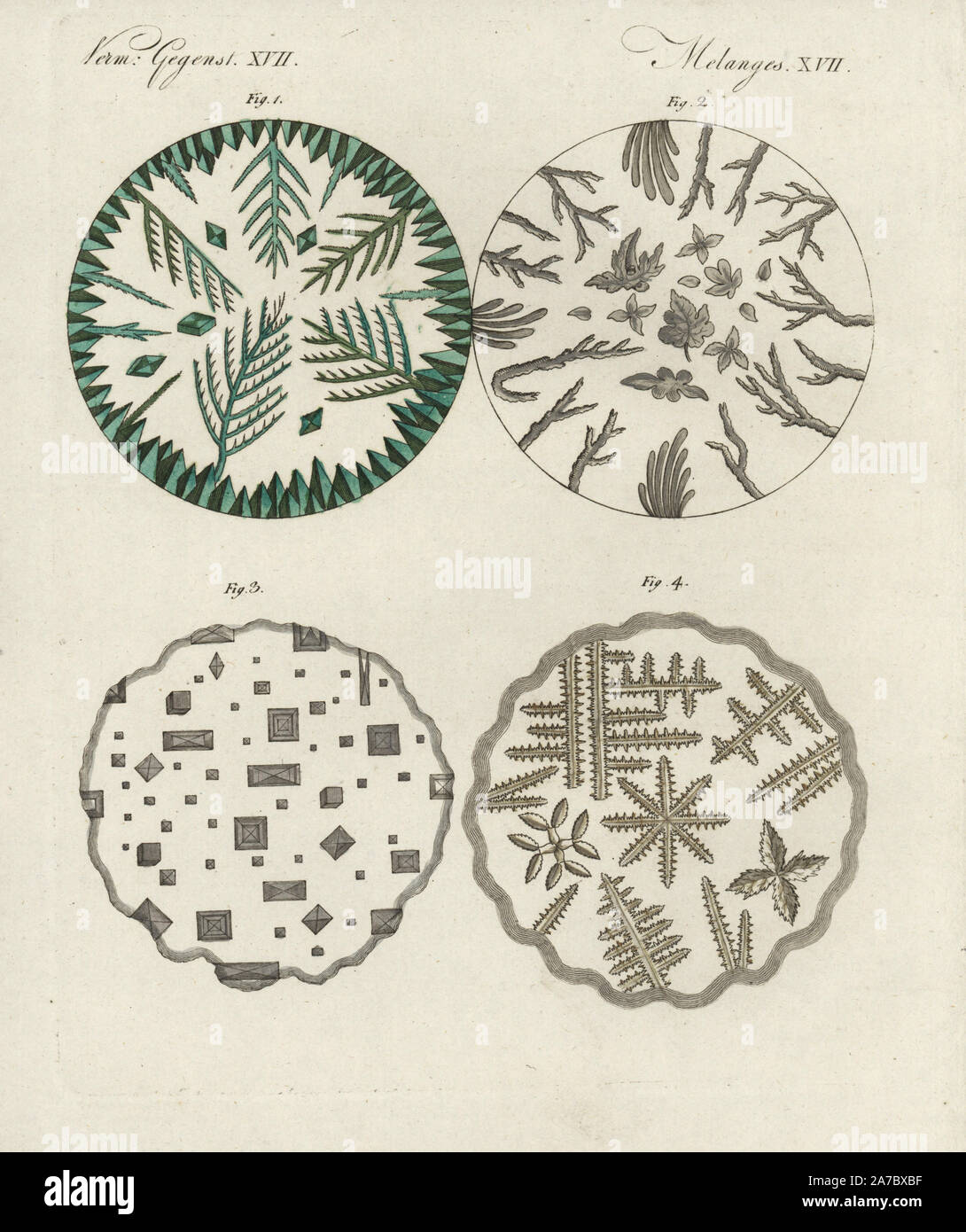 Crystals of verdigris 1, boric acid 2, common salt 3, and sal ammoniac 4 under the microscope. Handcoloured copperplate engraving from Bertuch's 'Bilderbuch fur Kinder' (Picture Book for Children), Weimar, 1798. Friedrich Johann Bertuch (1747-1822) was a German publisher and man of arts most famous for his 12-volume encyclopedia for children illustrated with 1,200 engraved plates on natural history, science, costume, mythology, etc., published from 1790-1830. Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/crystals-of-verdigris-1-boric-acid-2-common-salt-3-and-sal-ammoniac-4-under-the-microscope-handcoloured-copperplate-engraving-from-bertuchs-bilderbuch-fur-kinder-picture-book-for-children-weimar-1798-friedrich-johann-bertuch-1747-1822-was-a-german-publisher-and-man-of-arts-most-famous-for-his-12-volume-encyclopedia-for-children-illustrated-with-1200-engraved-plates-on-natural-history-science-costume-mythology-etc-published-from-1790-1830-image331561763.html
Crystals of verdigris 1, boric acid 2, common salt 3, and sal ammoniac 4 under the microscope. Handcoloured copperplate engraving from Bertuch's 'Bilderbuch fur Kinder' (Picture Book for Children), Weimar, 1798. Friedrich Johann Bertuch (1747-1822) was a German publisher and man of arts most famous for his 12-volume encyclopedia for children illustrated with 1,200 engraved plates on natural history, science, costume, mythology, etc., published from 1790-1830. Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/crystals-of-verdigris-1-boric-acid-2-common-salt-3-and-sal-ammoniac-4-under-the-microscope-handcoloured-copperplate-engraving-from-bertuchs-bilderbuch-fur-kinder-picture-book-for-children-weimar-1798-friedrich-johann-bertuch-1747-1822-was-a-german-publisher-and-man-of-arts-most-famous-for-his-12-volume-encyclopedia-for-children-illustrated-with-1200-engraved-plates-on-natural-history-science-costume-mythology-etc-published-from-1790-1830-image331561763.htmlRM2A7BXBF–Crystals of verdigris 1, boric acid 2, common salt 3, and sal ammoniac 4 under the microscope. Handcoloured copperplate engraving from Bertuch's 'Bilderbuch fur Kinder' (Picture Book for Children), Weimar, 1798. Friedrich Johann Bertuch (1747-1822) was a German publisher and man of arts most famous for his 12-volume encyclopedia for children illustrated with 1,200 engraved plates on natural history, science, costume, mythology, etc., published from 1790-1830.
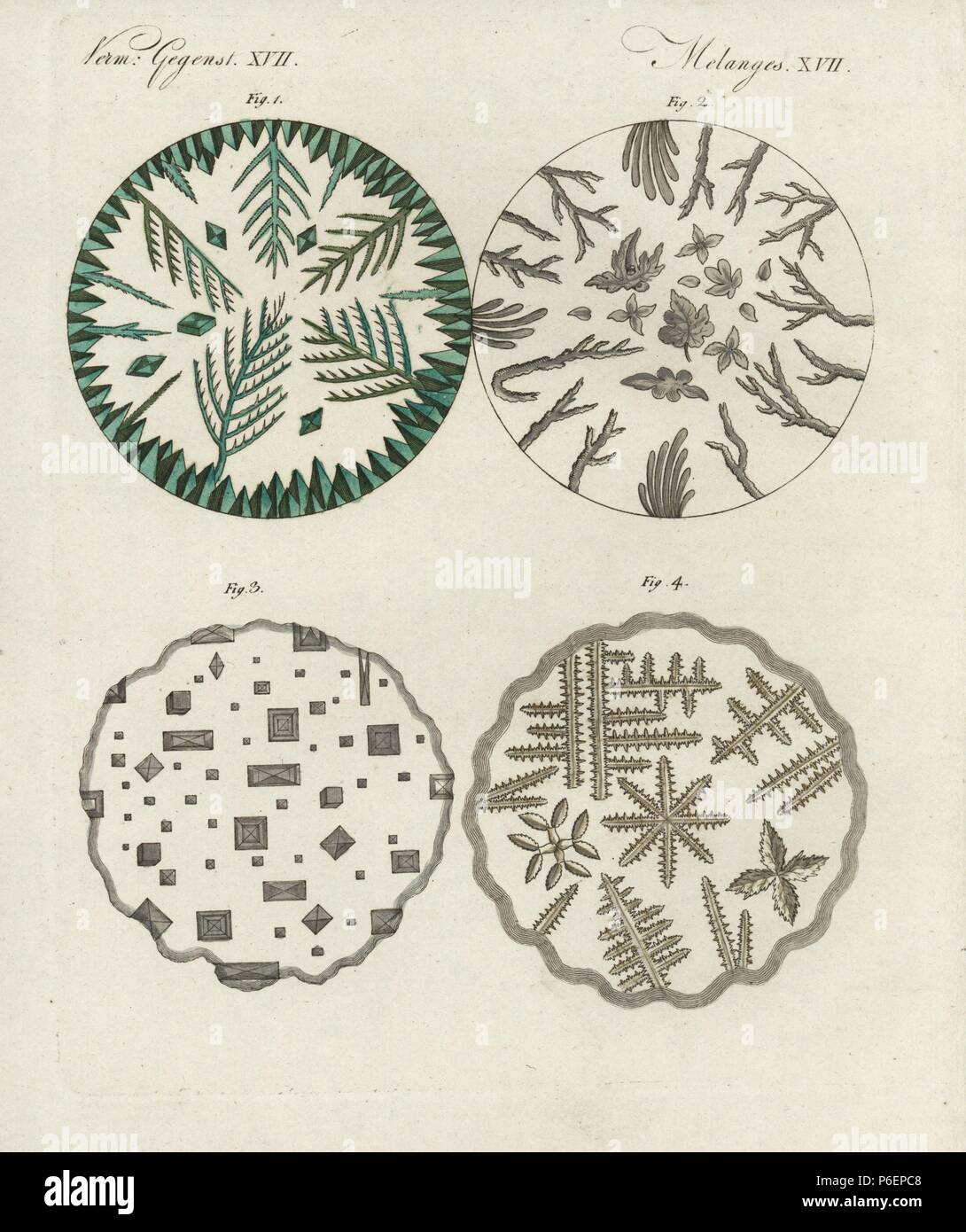 Crystals of verdigris 1, boric acid 2, common salt 3, and sal ammoniac 4 under the microscope. Handcoloured copperplate engraving from Bertuch's 'Bilderbuch fur Kinder' (Picture Book for Children), Weimar, 1798. Friedrich Johann Bertuch (1747-1822) was a German publisher and man of arts most famous for his 12-volume encyclopedia for children illustrated with 1,200 engraved plates on natural history, science, costume, mythology, etc., published from 1790-1830. Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/crystals-of-verdigris-1-boric-acid-2-common-salt-3-and-sal-ammoniac-4-under-the-microscope-handcoloured-copperplate-engraving-from-bertuchs-bilderbuch-fur-kinder-picture-book-for-children-weimar-1798-friedrich-johann-bertuch-1747-1822-was-a-german-publisher-and-man-of-arts-most-famous-for-his-12-volume-encyclopedia-for-children-illustrated-with-1200-engraved-plates-on-natural-history-science-costume-mythology-etc-published-from-1790-1830-image210537272.html
Crystals of verdigris 1, boric acid 2, common salt 3, and sal ammoniac 4 under the microscope. Handcoloured copperplate engraving from Bertuch's 'Bilderbuch fur Kinder' (Picture Book for Children), Weimar, 1798. Friedrich Johann Bertuch (1747-1822) was a German publisher and man of arts most famous for his 12-volume encyclopedia for children illustrated with 1,200 engraved plates on natural history, science, costume, mythology, etc., published from 1790-1830. Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/crystals-of-verdigris-1-boric-acid-2-common-salt-3-and-sal-ammoniac-4-under-the-microscope-handcoloured-copperplate-engraving-from-bertuchs-bilderbuch-fur-kinder-picture-book-for-children-weimar-1798-friedrich-johann-bertuch-1747-1822-was-a-german-publisher-and-man-of-arts-most-famous-for-his-12-volume-encyclopedia-for-children-illustrated-with-1200-engraved-plates-on-natural-history-science-costume-mythology-etc-published-from-1790-1830-image210537272.htmlRMP6EPC8–Crystals of verdigris 1, boric acid 2, common salt 3, and sal ammoniac 4 under the microscope. Handcoloured copperplate engraving from Bertuch's 'Bilderbuch fur Kinder' (Picture Book for Children), Weimar, 1798. Friedrich Johann Bertuch (1747-1822) was a German publisher and man of arts most famous for his 12-volume encyclopedia for children illustrated with 1,200 engraved plates on natural history, science, costume, mythology, etc., published from 1790-1830.
 Alchemy Alphabet: SAL-AMMONIAC (Sal Ammoniacus, Sal volatile), Sal ammiac, Salmiac, Sal armagnac (Salt armoniak), Nushadir salt. Ammonium chloride. Stock Vectorhttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/alchemy-alphabet-sal-ammoniac-sal-ammoniacus-sal-volatile-sal-ammiac-salmiac-sal-armagnac-salt-armoniak-nushadir-salt-ammonium-chloride-image415081525.html
Alchemy Alphabet: SAL-AMMONIAC (Sal Ammoniacus, Sal volatile), Sal ammiac, Salmiac, Sal armagnac (Salt armoniak), Nushadir salt. Ammonium chloride. Stock Vectorhttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/alchemy-alphabet-sal-ammoniac-sal-ammoniacus-sal-volatile-sal-ammiac-salmiac-sal-armagnac-salt-armoniak-nushadir-salt-ammonium-chloride-image415081525.htmlRF2F38GM5–Alchemy Alphabet: SAL-AMMONIAC (Sal Ammoniacus, Sal volatile), Sal ammiac, Salmiac, Sal armagnac (Salt armoniak), Nushadir salt. Ammonium chloride.
 Salmiac pastilles in front of white background Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/salmiac-pastilles-in-front-of-white-background-image335687077.html
Salmiac pastilles in front of white background Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/salmiac-pastilles-in-front-of-white-background-image335687077.htmlRF2AE3T85–Salmiac pastilles in front of white background
 Sal ammoniac. minerals Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/sal-ammoniac-minerals-image472958413.html
Sal ammoniac. minerals Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/sal-ammoniac-minerals-image472958413.htmlRM2JDD37W–Sal ammoniac. minerals
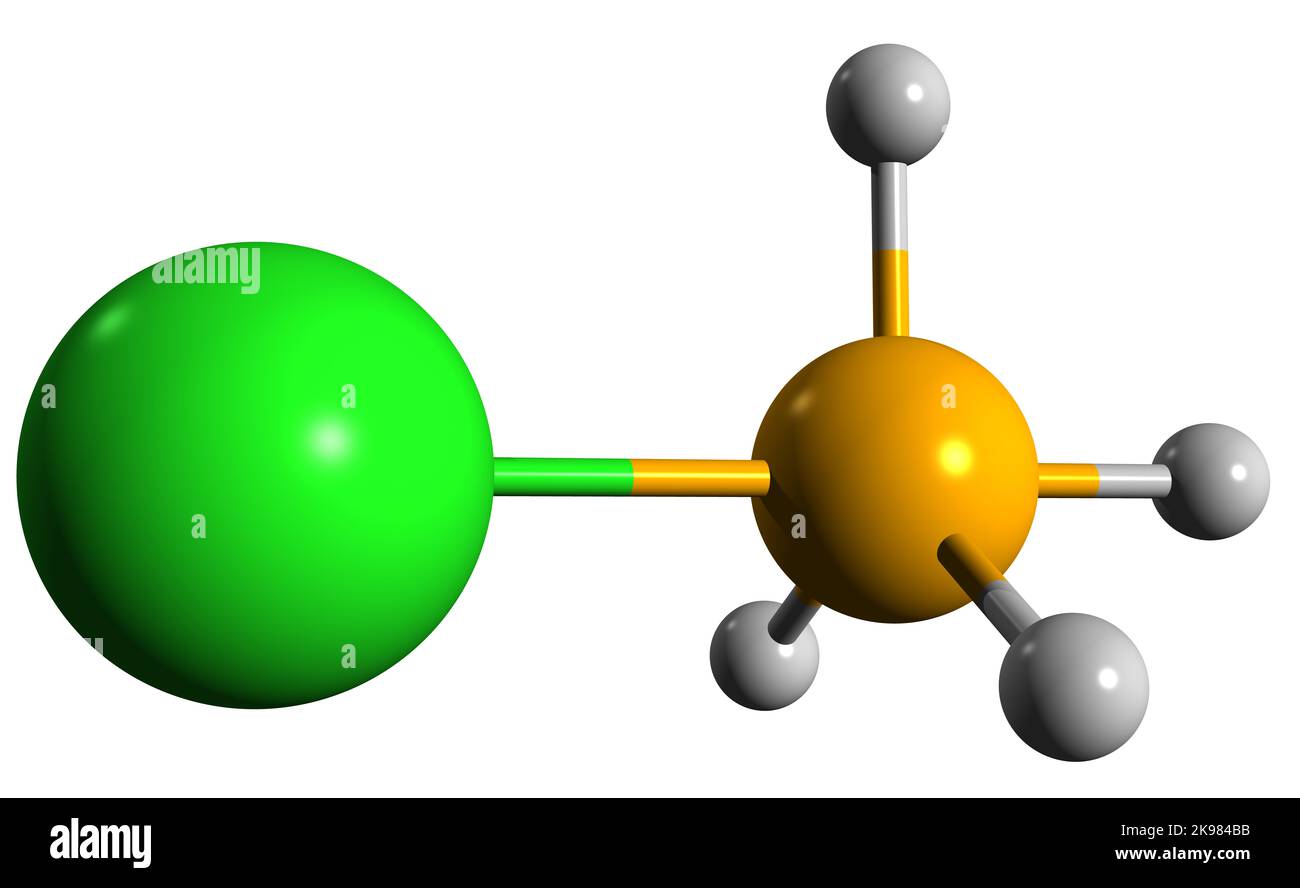 3D image of Ammonium chloride skeletal formula - molecular chemical structure of Sal ammoniac isolated on white background Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/3d-image-of-ammonium-chloride-skeletal-formula-molecular-chemical-structure-of-sal-ammoniac-isolated-on-white-background-image487601279.html
3D image of Ammonium chloride skeletal formula - molecular chemical structure of Sal ammoniac isolated on white background Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/3d-image-of-ammonium-chloride-skeletal-formula-molecular-chemical-structure-of-sal-ammoniac-isolated-on-white-background-image487601279.htmlRF2K984BB–3D image of Ammonium chloride skeletal formula - molecular chemical structure of Sal ammoniac isolated on white background
 Soldering iron is cleaned on a sal ammoniac stone Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/soldering-iron-is-cleaned-on-a-sal-ammoniac-stone-image553112396.html
Soldering iron is cleaned on a sal ammoniac stone Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/soldering-iron-is-cleaned-on-a-sal-ammoniac-stone-image553112396.htmlRF2R3TCE4–Soldering iron is cleaned on a sal ammoniac stone
 many black ammonia pastilles on a table Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/many-black-ammonia-pastilles-on-a-table-image336968832.html
many black ammonia pastilles on a table Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/many-black-ammonia-pastilles-on-a-table-image336968832.htmlRF2AG6754–many black ammonia pastilles on a table
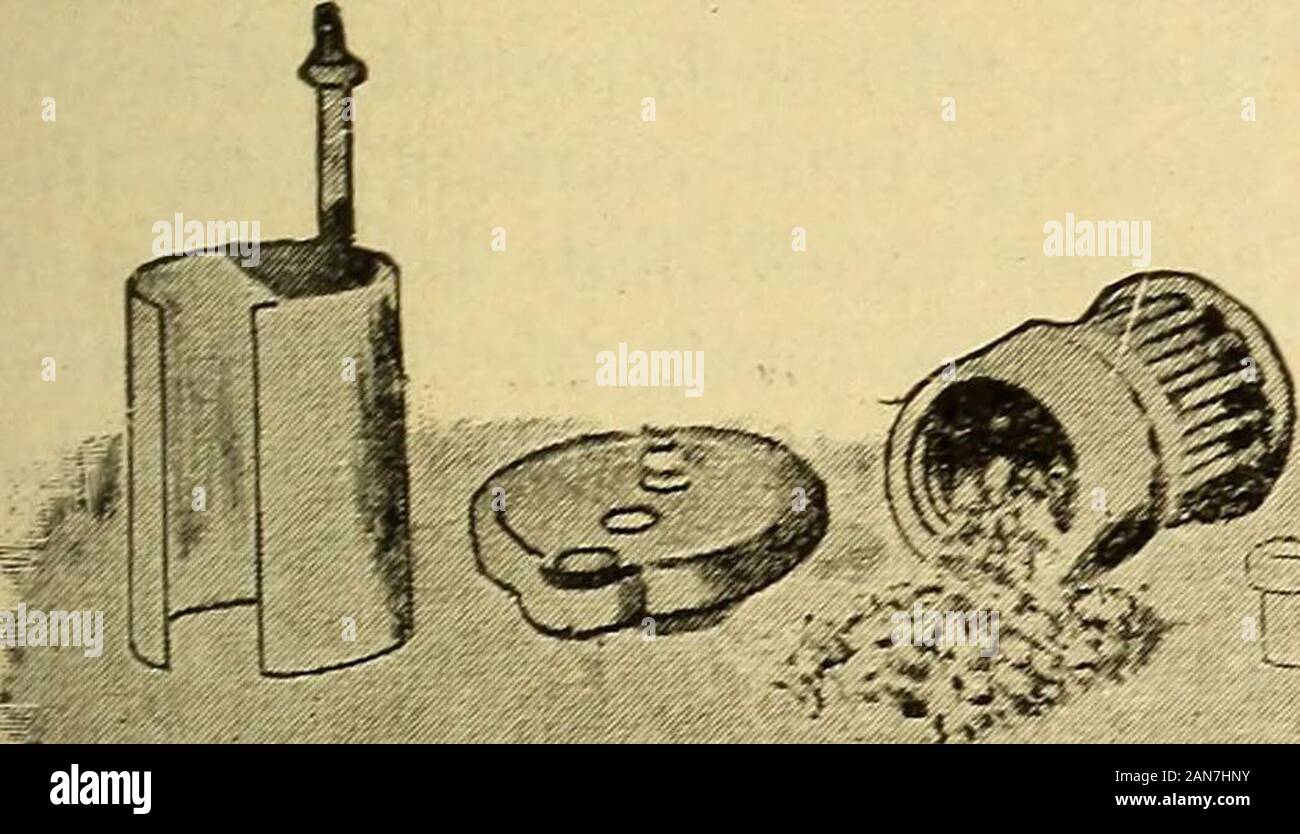 Lessons in practical electricity; principles, experiments, and arithmetical problems, an elementary text-book . Fig. 88.—Elements of a Carbon Cylinder Cell.Zinc, carbon and sal-ammoniac solution. BATTERIES. 73 carbon is madewith oxide of cell of the Standard Carbon Co., in which the in the form of a porous cup and then fillec manganese to prevent polarization. Still another form of the same make is shown in Fig. 90, in which the space between the two concentric carbon cylinders has been filled with oxide of. Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/lessons-in-practical-electricity-principles-experiments-and-arithmetical-problems-an-elementary-text-book-fig-88elements-of-a-carbon-cylinder-cellzinc-carbon-and-sal-ammoniac-solution-batteries-73-carbon-is-madewith-oxide-of-cell-of-the-standard-carbon-co-in-which-the-in-the-form-of-a-porous-cup-and-then-fillec-manganese-to-prevent-polarization-still-another-form-of-the-same-make-is-shown-in-fig-90-in-which-the-space-between-the-two-concentric-carbon-cylinders-has-been-filled-with-oxide-of-image340072375.html
Lessons in practical electricity; principles, experiments, and arithmetical problems, an elementary text-book . Fig. 88.—Elements of a Carbon Cylinder Cell.Zinc, carbon and sal-ammoniac solution. BATTERIES. 73 carbon is madewith oxide of cell of the Standard Carbon Co., in which the in the form of a porous cup and then fillec manganese to prevent polarization. Still another form of the same make is shown in Fig. 90, in which the space between the two concentric carbon cylinders has been filled with oxide of. Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/lessons-in-practical-electricity-principles-experiments-and-arithmetical-problems-an-elementary-text-book-fig-88elements-of-a-carbon-cylinder-cellzinc-carbon-and-sal-ammoniac-solution-batteries-73-carbon-is-madewith-oxide-of-cell-of-the-standard-carbon-co-in-which-the-in-the-form-of-a-porous-cup-and-then-fillec-manganese-to-prevent-polarization-still-another-form-of-the-same-make-is-shown-in-fig-90-in-which-the-space-between-the-two-concentric-carbon-cylinders-has-been-filled-with-oxide-of-image340072375.htmlRM2AN7HNY–Lessons in practical electricity; principles, experiments, and arithmetical problems, an elementary text-book . Fig. 88.—Elements of a Carbon Cylinder Cell.Zinc, carbon and sal-ammoniac solution. BATTERIES. 73 carbon is madewith oxide of cell of the Standard Carbon Co., in which the in the form of a porous cup and then fillec manganese to prevent polarization. Still another form of the same make is shown in Fig. 90, in which the space between the two concentric carbon cylinders has been filled with oxide of.
 Productions of the Solfaterra, 1776. Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/productions-of-the-solfaterra-1776-image186152221.html
Productions of the Solfaterra, 1776. Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/productions-of-the-solfaterra-1776-image186152221.htmlRMMPRY1H–Productions of the Solfaterra, 1776.
 Chemistry Appliances Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/stock-photo-chemistry-appliances-105325320.html
Chemistry Appliances Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/stock-photo-chemistry-appliances-105325320.htmlRMG39YEG–Chemistry Appliances
 A bag of Jämtgott brand Dragster 3000 Swedish salty licorice candy isolated on a white background. licorice coated with ammonium chloride. Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/a-bag-of-jmtgott-brand-dragster-3000-swedish-salty-licorice-candy-isolated-on-a-white-background-licorice-coated-with-ammonium-chloride-image449856173.html
A bag of Jämtgott brand Dragster 3000 Swedish salty licorice candy isolated on a white background. licorice coated with ammonium chloride. Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/a-bag-of-jmtgott-brand-dragster-3000-swedish-salty-licorice-candy-isolated-on-a-white-background-licorice-coated-with-ammonium-chloride-image449856173.htmlRM2H3TM3W–A bag of Jämtgott brand Dragster 3000 Swedish salty licorice candy isolated on a white background. licorice coated with ammonium chloride.
 LIST OF PATENT CLAIMS Issued from the United States Patent Office MODE OF GRINDING PUPPET VALVES WHILE THE RE-ISSUE. DESIGN. Sal Ammoniac. Extension of a Patent. Snake Bites., scientific american, 1852-07-17 Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/list-of-patent-claims-issued-from-the-united-states-patent-office-mode-of-grinding-puppet-valves-while-the-re-issue-design-sal-ammoniac-extension-of-a-patent-snake-bites-scientific-american-1852-07-17-image334300255.html
LIST OF PATENT CLAIMS Issued from the United States Patent Office MODE OF GRINDING PUPPET VALVES WHILE THE RE-ISSUE. DESIGN. Sal Ammoniac. Extension of a Patent. Snake Bites., scientific american, 1852-07-17 Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/list-of-patent-claims-issued-from-the-united-states-patent-office-mode-of-grinding-puppet-valves-while-the-re-issue-design-sal-ammoniac-extension-of-a-patent-snake-bites-scientific-american-1852-07-17-image334300255.htmlRM2ABTKAR–LIST OF PATENT CLAIMS Issued from the United States Patent Office MODE OF GRINDING PUPPET VALVES WHILE THE RE-ISSUE. DESIGN. Sal Ammoniac. Extension of a Patent. Snake Bites., scientific american, 1852-07-17
 . De re metallica. Metallurgy; Mineral industries. BOOK X. 455 Those ingredients above are peculiar to each cement, but what follows is common to all. Each of the ingredients is first separately crushed to powder ; the bricks are placed on a hard rock or marble, and crushed with an iron implement ; the other things are crushed in a mortar with a pestle ; each is separately passed through a sieve. Then they arc all mixed together, and are moistened with vinegar in which a little sal-ammoniac has been dissolved, if the cement does not contain any. But some workers, however, prefer to moisten the Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/de-re-metallica-metallurgy-mineral-industries-book-x-455-those-ingredients-above-are-peculiar-to-each-cement-but-what-follows-is-common-to-all-each-of-the-ingredients-is-first-separately-crushed-to-powder-the-bricks-are-placed-on-a-hard-rock-or-marble-and-crushed-with-an-iron-implement-the-other-things-are-crushed-in-a-mortar-with-a-pestle-each-is-separately-passed-through-a-sieve-then-they-arc-all-mixed-together-and-are-moistened-with-vinegar-in-which-a-little-sal-ammoniac-has-been-dissolved-if-the-cement-does-not-contain-any-but-some-workers-however-prefer-to-moisten-the-image216003548.html
. De re metallica. Metallurgy; Mineral industries. BOOK X. 455 Those ingredients above are peculiar to each cement, but what follows is common to all. Each of the ingredients is first separately crushed to powder ; the bricks are placed on a hard rock or marble, and crushed with an iron implement ; the other things are crushed in a mortar with a pestle ; each is separately passed through a sieve. Then they arc all mixed together, and are moistened with vinegar in which a little sal-ammoniac has been dissolved, if the cement does not contain any. But some workers, however, prefer to moisten the Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/de-re-metallica-metallurgy-mineral-industries-book-x-455-those-ingredients-above-are-peculiar-to-each-cement-but-what-follows-is-common-to-all-each-of-the-ingredients-is-first-separately-crushed-to-powder-the-bricks-are-placed-on-a-hard-rock-or-marble-and-crushed-with-an-iron-implement-the-other-things-are-crushed-in-a-mortar-with-a-pestle-each-is-separately-passed-through-a-sieve-then-they-arc-all-mixed-together-and-are-moistened-with-vinegar-in-which-a-little-sal-ammoniac-has-been-dissolved-if-the-cement-does-not-contain-any-but-some-workers-however-prefer-to-moisten-the-image216003548.htmlRMPFBPMC–. De re metallica. Metallurgy; Mineral industries. BOOK X. 455 Those ingredients above are peculiar to each cement, but what follows is common to all. Each of the ingredients is first separately crushed to powder ; the bricks are placed on a hard rock or marble, and crushed with an iron implement ; the other things are crushed in a mortar with a pestle ; each is separately passed through a sieve. Then they arc all mixed together, and are moistened with vinegar in which a little sal-ammoniac has been dissolved, if the cement does not contain any. But some workers, however, prefer to moisten the
 Miscellaneous Notes and Receipts. The latter (spirits of sal-ammoniac) was used for the TEE '98 SOLAR ACETYLENE GAS BICYCLE LAMP. AN ACETYLENE GAS BICYCLE LAMP. thrown out and the lumps retained and the screen Patents that Pay. A Balloon for War Purposes. plan is to fit it with search lights and a telephone. ACETYLENE GAS LAMP., scientific american, 1898-04-23 Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/miscellaneous-notes-and-receipts-the-latter-spirits-of-sal-ammoniac-was-used-for-the-tee-98-solar-acetylene-gas-bicycle-lamp-an-acetylene-gas-bicycle-lamp-thrown-out-and-the-lumps-retained-and-the-screen-patents-that-pay-a-balloon-for-war-purposes-plan-is-to-fit-it-with-search-lights-and-a-telephone-acetylene-gas-lamp-scientific-american-1898-04-23-image334342178.html
Miscellaneous Notes and Receipts. The latter (spirits of sal-ammoniac) was used for the TEE '98 SOLAR ACETYLENE GAS BICYCLE LAMP. AN ACETYLENE GAS BICYCLE LAMP. thrown out and the lumps retained and the screen Patents that Pay. A Balloon for War Purposes. plan is to fit it with search lights and a telephone. ACETYLENE GAS LAMP., scientific american, 1898-04-23 Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/miscellaneous-notes-and-receipts-the-latter-spirits-of-sal-ammoniac-was-used-for-the-tee-98-solar-acetylene-gas-bicycle-lamp-an-acetylene-gas-bicycle-lamp-thrown-out-and-the-lumps-retained-and-the-screen-patents-that-pay-a-balloon-for-war-purposes-plan-is-to-fit-it-with-search-lights-and-a-telephone-acetylene-gas-lamp-scientific-american-1898-04-23-image334342178.htmlRM2ABXGT2–Miscellaneous Notes and Receipts. The latter (spirits of sal-ammoniac) was used for the TEE '98 SOLAR ACETYLENE GAS BICYCLE LAMP. AN ACETYLENE GAS BICYCLE LAMP. thrown out and the lumps retained and the screen Patents that Pay. A Balloon for War Purposes. plan is to fit it with search lights and a telephone. ACETYLENE GAS LAMP., scientific american, 1898-04-23
 Archive image from page 492 of De re metallica (1950). De re metallica deremetallica50agri Year: 1950 BOOK X. 455 Those ingredients above are peculiar to each cement, but what follows is common to all. Each of the ingredients is first separately crushed to powder ; the bricks are placed on a hard rock or marble, and crushed with an iron implement ; the other things are crushed in a mortar with a pestle ; each is separately passed through a sieve. Then they arc all mixed together, and are moistened with vinegar in which a little sal-ammoniac has been dissolved, if the cement does not contain a Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/archive-image-from-page-492-of-de-re-metallica-1950-de-re-metallica-deremetallica50agri-year-1950-book-x-455-those-ingredients-above-are-peculiar-to-each-cement-but-what-follows-is-common-to-all-each-of-the-ingredients-is-first-separately-crushed-to-powder-the-bricks-are-placed-on-a-hard-rock-or-marble-and-crushed-with-an-iron-implement-the-other-things-are-crushed-in-a-mortar-with-a-pestle-each-is-separately-passed-through-a-sieve-then-they-arc-all-mixed-together-and-are-moistened-with-vinegar-in-which-a-little-sal-ammoniac-has-been-dissolved-if-the-cement-does-not-contain-a-image258677063.html
Archive image from page 492 of De re metallica (1950). De re metallica deremetallica50agri Year: 1950 BOOK X. 455 Those ingredients above are peculiar to each cement, but what follows is common to all. Each of the ingredients is first separately crushed to powder ; the bricks are placed on a hard rock or marble, and crushed with an iron implement ; the other things are crushed in a mortar with a pestle ; each is separately passed through a sieve. Then they arc all mixed together, and are moistened with vinegar in which a little sal-ammoniac has been dissolved, if the cement does not contain a Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/archive-image-from-page-492-of-de-re-metallica-1950-de-re-metallica-deremetallica50agri-year-1950-book-x-455-those-ingredients-above-are-peculiar-to-each-cement-but-what-follows-is-common-to-all-each-of-the-ingredients-is-first-separately-crushed-to-powder-the-bricks-are-placed-on-a-hard-rock-or-marble-and-crushed-with-an-iron-implement-the-other-things-are-crushed-in-a-mortar-with-a-pestle-each-is-separately-passed-through-a-sieve-then-they-arc-all-mixed-together-and-are-moistened-with-vinegar-in-which-a-little-sal-ammoniac-has-been-dissolved-if-the-cement-does-not-contain-a-image258677063.htmlRMW0RN6F–Archive image from page 492 of De re metallica (1950). De re metallica deremetallica50agri Year: 1950 BOOK X. 455 Those ingredients above are peculiar to each cement, but what follows is common to all. Each of the ingredients is first separately crushed to powder ; the bricks are placed on a hard rock or marble, and crushed with an iron implement ; the other things are crushed in a mortar with a pestle ; each is separately passed through a sieve. Then they arc all mixed together, and are moistened with vinegar in which a little sal-ammoniac has been dissolved, if the cement does not contain a
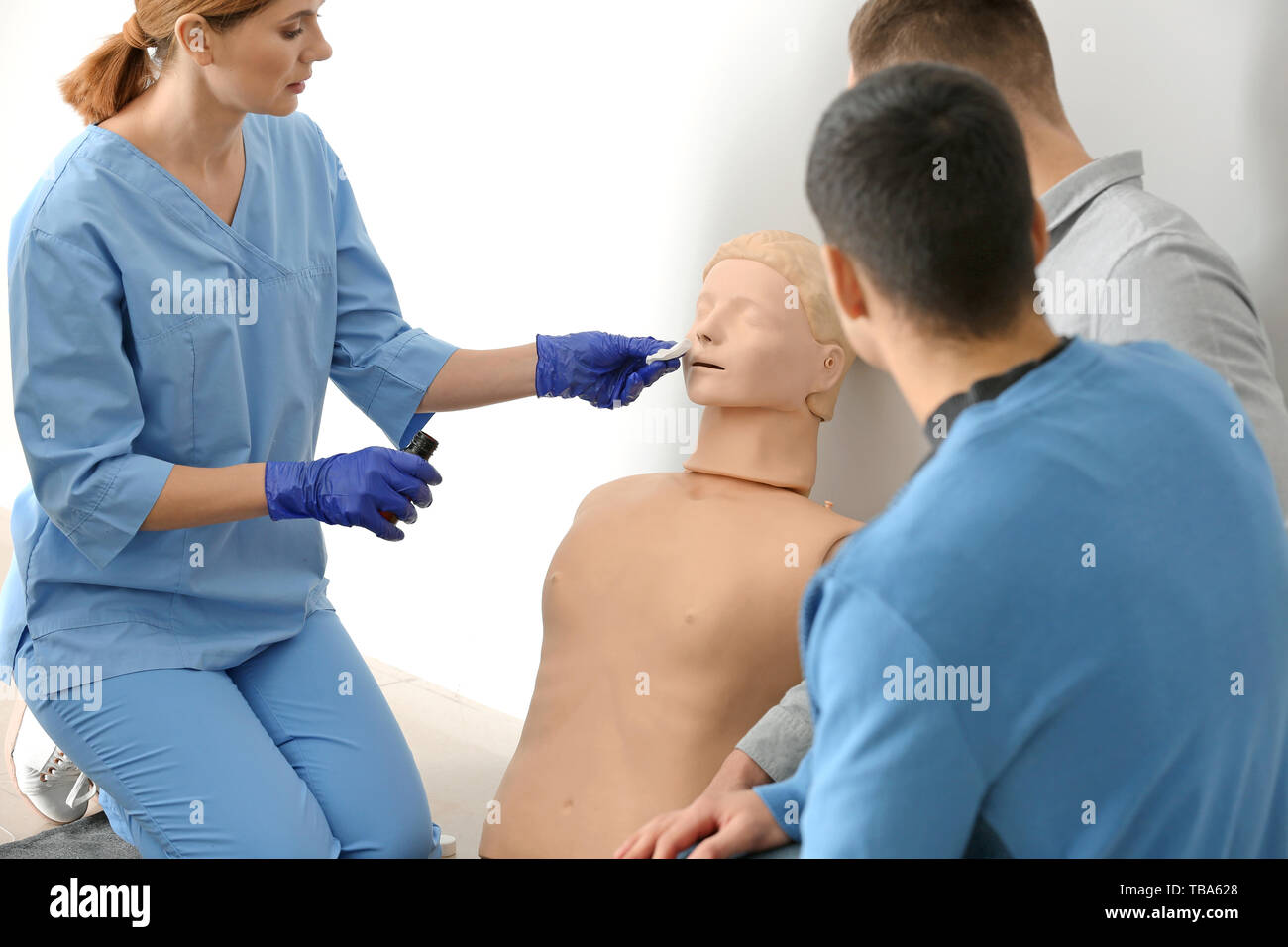 Group of people with instructor at first aid training course Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/group-of-people-with-instructor-at-first-aid-training-course-image247930656.html
Group of people with instructor at first aid training course Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/group-of-people-with-instructor-at-first-aid-training-course-image247930656.htmlRFTBA628–Group of people with instructor at first aid training course
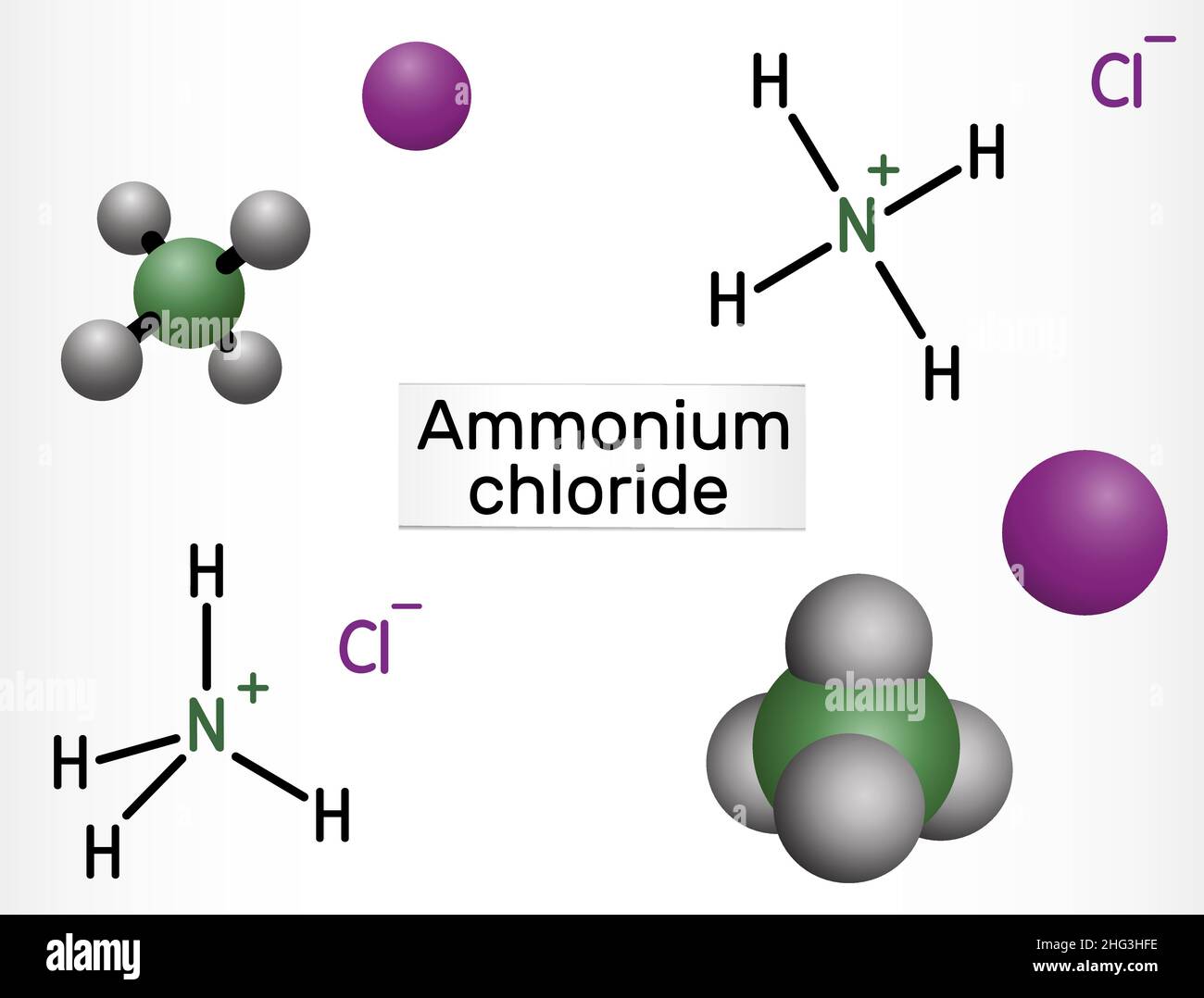 Ammonium chloride, NH4Cl molecule. It is inorganic compound, food supplement E510, used as fertilizer and a flavouring agent. Structural chemical form Stock Vectorhttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/ammonium-chloride-nh4cl-molecule-it-is-inorganic-compound-food-supplement-e510-used-as-fertilizer-and-a-flavouring-agent-structural-chemical-form-image457383682.html
Ammonium chloride, NH4Cl molecule. It is inorganic compound, food supplement E510, used as fertilizer and a flavouring agent. Structural chemical form Stock Vectorhttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/ammonium-chloride-nh4cl-molecule-it-is-inorganic-compound-food-supplement-e510-used-as-fertilizer-and-a-flavouring-agent-structural-chemical-form-image457383682.htmlRF2HG3HFE–Ammonium chloride, NH4Cl molecule. It is inorganic compound, food supplement E510, used as fertilizer and a flavouring agent. Structural chemical form
![Alchemy Alphabet: SAL-AMMONIAC (Sal Ammoniacus, Sal volatile), Sal ammiac, Salmiac, eq.: Nushadir salt. Ammonium chloride: Chemical formula=[NH₄Cl]. Stock Vector Alchemy Alphabet: SAL-AMMONIAC (Sal Ammoniacus, Sal volatile), Sal ammiac, Salmiac, eq.: Nushadir salt. Ammonium chloride: Chemical formula=[NH₄Cl]. Stock Vector](https://c8.alamy.com/comp/2FFBDMY/alchemy-alphabet-sal-ammoniac-sal-ammoniacus-sal-volatile-sal-ammiac-salmiac-eq-nushadir-salt-ammonium-chloride-chemical-formula=-nhcl-2FFBDMY.jpg) Alchemy Alphabet: SAL-AMMONIAC (Sal Ammoniacus, Sal volatile), Sal ammiac, Salmiac, eq.: Nushadir salt. Ammonium chloride: Chemical formula=[NH₄Cl]. Stock Vectorhttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/alchemy-alphabet-sal-ammoniac-sal-ammoniacus-sal-volatile-sal-ammiac-salmiac-eq-nushadir-salt-ammonium-chloride-chemical-formula=-nhcl-image422520923.html
Alchemy Alphabet: SAL-AMMONIAC (Sal Ammoniacus, Sal volatile), Sal ammiac, Salmiac, eq.: Nushadir salt. Ammonium chloride: Chemical formula=[NH₄Cl]. Stock Vectorhttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/alchemy-alphabet-sal-ammoniac-sal-ammoniacus-sal-volatile-sal-ammiac-salmiac-eq-nushadir-salt-ammonium-chloride-chemical-formula=-nhcl-image422520923.htmlRF2FFBDMY–Alchemy Alphabet: SAL-AMMONIAC (Sal Ammoniacus, Sal volatile), Sal ammiac, Salmiac, eq.: Nushadir salt. Ammonium chloride: Chemical formula=[NH₄Cl].
 Salmiac pastilles in a closeup Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/salmiac-pastilles-in-a-closeup-image351455106.html
Salmiac pastilles in a closeup Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/salmiac-pastilles-in-a-closeup-image351455106.htmlRF2BBP4G2–Salmiac pastilles in a closeup
 Sal ammoniac. minerals. Europe; Germany; Drittweiler Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/sal-ammoniac-minerals-europe-germany-drittweiler-image472969759.html
Sal ammoniac. minerals. Europe; Germany; Drittweiler Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/sal-ammoniac-minerals-europe-germany-drittweiler-image472969759.htmlRM2JDDHN3–Sal ammoniac. minerals. Europe; Germany; Drittweiler
 Soldering iron is cleaned on a sal ammoniac stone Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/soldering-iron-is-cleaned-on-a-sal-ammoniac-stone-image553112405.html
Soldering iron is cleaned on a sal ammoniac stone Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/soldering-iron-is-cleaned-on-a-sal-ammoniac-stone-image553112405.htmlRF2R3TCED–Soldering iron is cleaned on a sal ammoniac stone
 many black ammonia pastilles on a table Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/many-black-ammonia-pastilles-on-a-table-image336968839.html
many black ammonia pastilles on a table Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/many-black-ammonia-pastilles-on-a-table-image336968839.htmlRF2AG675B–many black ammonia pastilles on a table
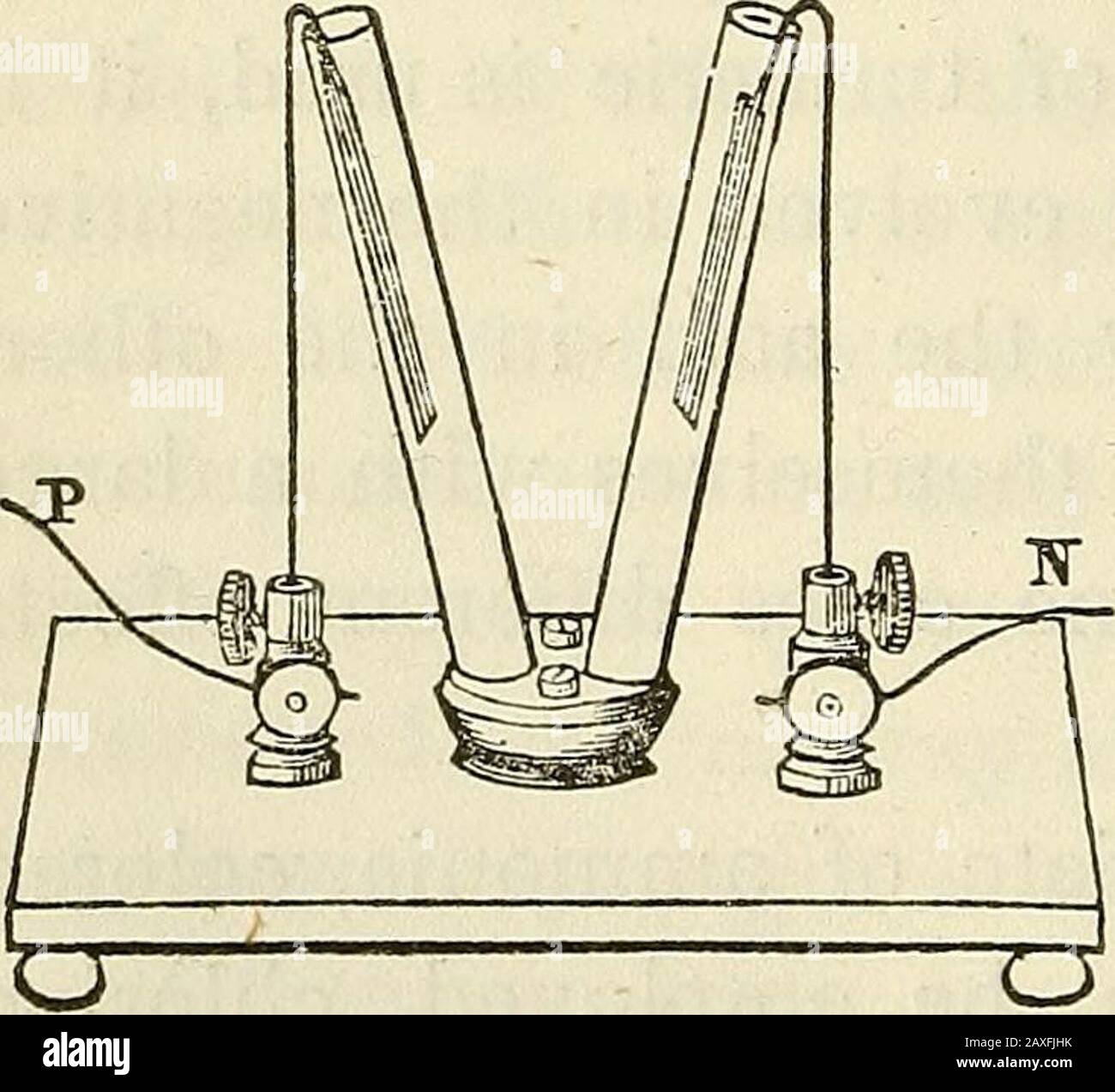 Davis's manual of magnetism : including galvanism, magnetism, electro-magnetism, electro-dynamics, magneto-electricity, and thermo-electricity . ygen shouldhave been liberated in the other. The chlorineappears therefore to be a secondary product, set freeby the combination of the hydrogen of the muriaticacid with oxygen, to form water. Or, according tothe view which regards sal-ammoniac as a chloride ofammonium, chlorine is directly liberated at the posi-tive pole; and at the negative, ammonium, which,not being able to exist in an isolated state, is in-stantly resolved into its components, amm Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/daviss-manual-of-magnetism-including-galvanism-magnetism-electro-magnetism-electro-dynamics-magneto-electricity-and-thermo-electricity-ygen-shouldhave-been-liberated-in-the-other-the-chlorineappears-therefore-to-be-a-secondary-product-set-freeby-the-combination-of-the-hydrogen-of-the-muriaticacid-with-oxygen-to-form-water-or-according-tothe-view-which-regards-sal-ammoniac-as-a-chloride-ofammonium-chlorine-is-directly-liberated-at-the-posi-tive-pole-and-at-the-negative-ammonium-whichnot-being-able-to-exist-in-an-isolated-state-is-in-stantly-resolved-into-its-components-amm-image343321935.html
Davis's manual of magnetism : including galvanism, magnetism, electro-magnetism, electro-dynamics, magneto-electricity, and thermo-electricity . ygen shouldhave been liberated in the other. The chlorineappears therefore to be a secondary product, set freeby the combination of the hydrogen of the muriaticacid with oxygen, to form water. Or, according tothe view which regards sal-ammoniac as a chloride ofammonium, chlorine is directly liberated at the posi-tive pole; and at the negative, ammonium, which,not being able to exist in an isolated state, is in-stantly resolved into its components, amm Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/daviss-manual-of-magnetism-including-galvanism-magnetism-electro-magnetism-electro-dynamics-magneto-electricity-and-thermo-electricity-ygen-shouldhave-been-liberated-in-the-other-the-chlorineappears-therefore-to-be-a-secondary-product-set-freeby-the-combination-of-the-hydrogen-of-the-muriaticacid-with-oxygen-to-form-water-or-according-tothe-view-which-regards-sal-ammoniac-as-a-chloride-ofammonium-chlorine-is-directly-liberated-at-the-posi-tive-pole-and-at-the-negative-ammonium-whichnot-being-able-to-exist-in-an-isolated-state-is-in-stantly-resolved-into-its-components-amm-image343321935.htmlRM2AXFJHK–Davis's manual of magnetism : including galvanism, magnetism, electro-magnetism, electro-dynamics, magneto-electricity, and thermo-electricity . ygen shouldhave been liberated in the other. The chlorineappears therefore to be a secondary product, set freeby the combination of the hydrogen of the muriaticacid with oxygen, to form water. Or, according tothe view which regards sal-ammoniac as a chloride ofammonium, chlorine is directly liberated at the posi-tive pole; and at the negative, ammonium, which,not being able to exist in an isolated state, is in-stantly resolved into its components, amm
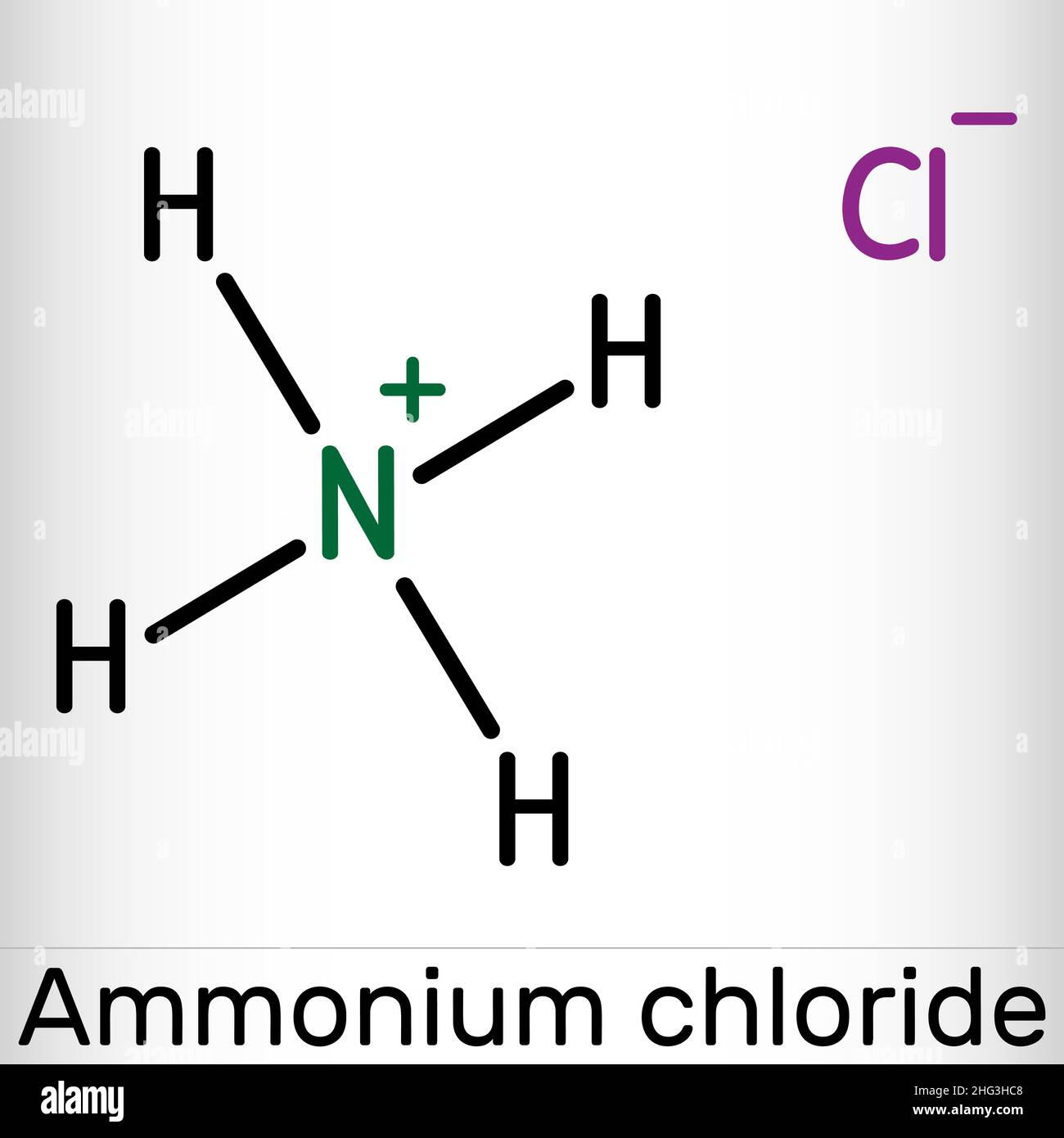 Ammonium chloride, NH4Cl molecule. It is inorganic compound, food supplement E510, used as fertilizer and a flavouring agent. Skeletal chemical formul Stock Vectorhttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/ammonium-chloride-nh4cl-molecule-it-is-inorganic-compound-food-supplement-e510-used-as-fertilizer-and-a-flavouring-agent-skeletal-chemical-formul-image457383592.html
Ammonium chloride, NH4Cl molecule. It is inorganic compound, food supplement E510, used as fertilizer and a flavouring agent. Skeletal chemical formul Stock Vectorhttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/ammonium-chloride-nh4cl-molecule-it-is-inorganic-compound-food-supplement-e510-used-as-fertilizer-and-a-flavouring-agent-skeletal-chemical-formul-image457383592.htmlRF2HG3HC8–Ammonium chloride, NH4Cl molecule. It is inorganic compound, food supplement E510, used as fertilizer and a flavouring agent. Skeletal chemical formul
 Salmiac pastilles in a closeup Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/salmiac-pastilles-in-a-closeup-image335687084.html
Salmiac pastilles in a closeup Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/salmiac-pastilles-in-a-closeup-image335687084.htmlRF2AE3T8C–Salmiac pastilles in a closeup
 Sal ammoniac. minerals. North America; Mexico; Paricutin Volcano Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/sal-ammoniac-minerals-north-america-mexico-paricutin-volcano-image472968317.html
Sal ammoniac. minerals. North America; Mexico; Paricutin Volcano Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/sal-ammoniac-minerals-north-america-mexico-paricutin-volcano-image472968317.htmlRM2JDDFWH–Sal ammoniac. minerals. North America; Mexico; Paricutin Volcano
 . Elements of chemistry ... e desire to make some experimentson ammonia, a gas which is rapidly absorbedby water and specifically lighter thanatmospheric air. The materials for separating this gas aremuriate of ammonia, called also sal ammoniac, and slackedquich-lime. These being separately reduced to powder,equal parts are then mixed, and introduced into the flask a,and the tube put into its place. On application of a gentleheat, the gas will be set free in consequence of the combi-nation which takes place between the lime and the muriaticacid of the muriate of ammonia. The ammonia is thus se Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/elements-of-chemistry-e-desire-to-make-some-experimentson-ammonia-a-gas-which-is-rapidly-absorbedby-water-and-specifically-lighter-thanatmospheric-air-the-materials-for-separating-this-gas-aremuriate-of-ammonia-called-also-sal-ammoniac-and-slackedquich-lime-these-being-separately-reduced-to-powderequal-parts-are-then-mixed-and-introduced-into-the-flask-aand-the-tube-put-into-its-place-on-application-of-a-gentleheat-the-gas-will-be-set-free-in-consequence-of-the-combi-nation-which-takes-place-between-the-lime-and-the-muriaticacid-of-the-muriate-of-ammonia-the-ammonia-is-thus-se-image337143197.html
. Elements of chemistry ... e desire to make some experimentson ammonia, a gas which is rapidly absorbedby water and specifically lighter thanatmospheric air. The materials for separating this gas aremuriate of ammonia, called also sal ammoniac, and slackedquich-lime. These being separately reduced to powder,equal parts are then mixed, and introduced into the flask a,and the tube put into its place. On application of a gentleheat, the gas will be set free in consequence of the combi-nation which takes place between the lime and the muriaticacid of the muriate of ammonia. The ammonia is thus se Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/elements-of-chemistry-e-desire-to-make-some-experimentson-ammonia-a-gas-which-is-rapidly-absorbedby-water-and-specifically-lighter-thanatmospheric-air-the-materials-for-separating-this-gas-aremuriate-of-ammonia-called-also-sal-ammoniac-and-slackedquich-lime-these-being-separately-reduced-to-powderequal-parts-are-then-mixed-and-introduced-into-the-flask-aand-the-tube-put-into-its-place-on-application-of-a-gentleheat-the-gas-will-be-set-free-in-consequence-of-the-combi-nation-which-takes-place-between-the-lime-and-the-muriaticacid-of-the-muriate-of-ammonia-the-ammonia-is-thus-se-image337143197.htmlRM2AGE5GD–. Elements of chemistry ... e desire to make some experimentson ammonia, a gas which is rapidly absorbedby water and specifically lighter thanatmospheric air. The materials for separating this gas aremuriate of ammonia, called also sal ammoniac, and slackedquich-lime. These being separately reduced to powder,equal parts are then mixed, and introduced into the flask a,and the tube put into its place. On application of a gentleheat, the gas will be set free in consequence of the combi-nation which takes place between the lime and the muriaticacid of the muriate of ammonia. The ammonia is thus se
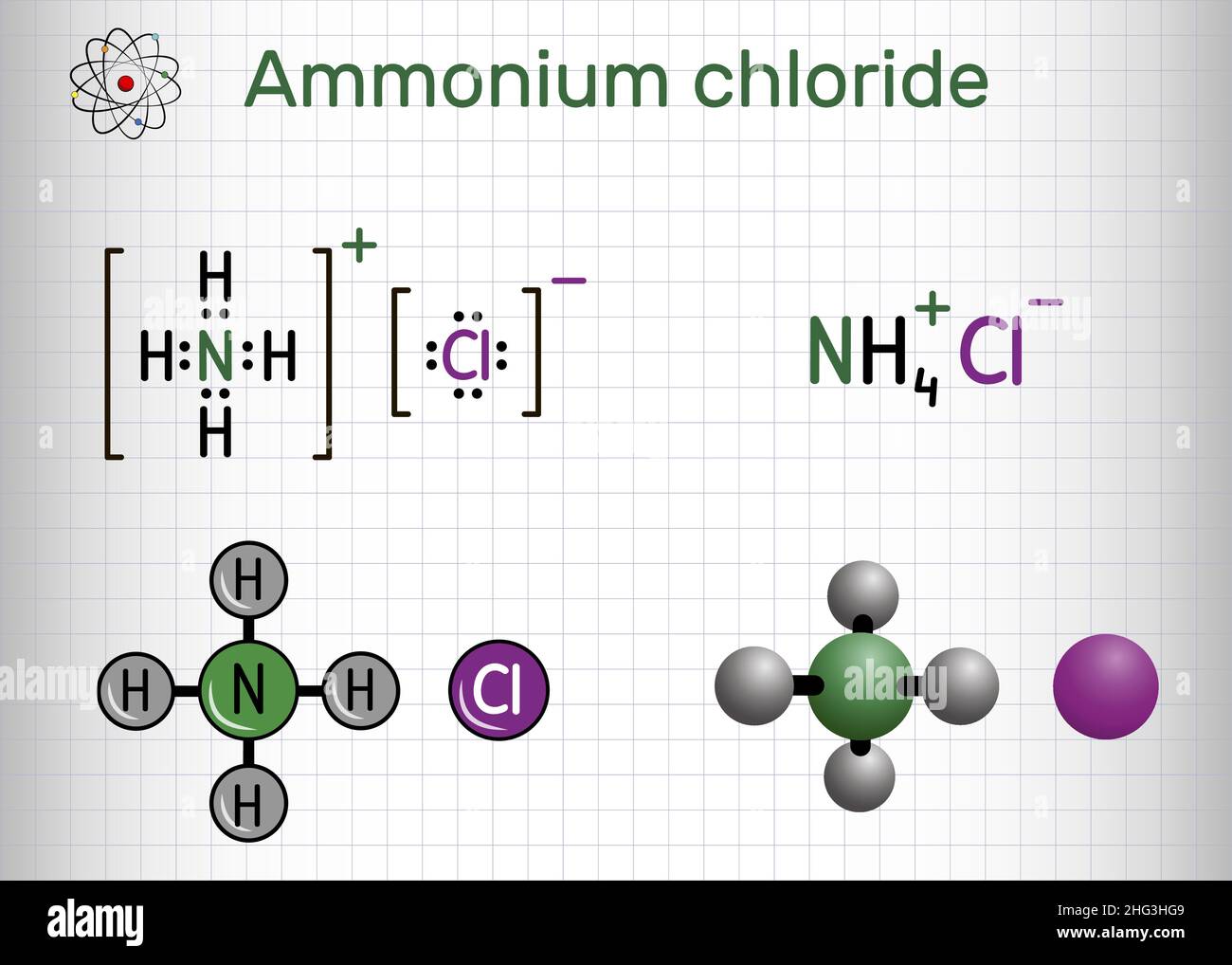 Ammonium chloride, NH4Cl molecule. It is inorganic compound, food supplement E510, used as fertilizer, flavouring agent. Structural formula, molecule Stock Vectorhttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/ammonium-chloride-nh4cl-molecule-it-is-inorganic-compound-food-supplement-e510-used-as-fertilizer-flavouring-agent-structural-formula-molecule-image457383705.html
Ammonium chloride, NH4Cl molecule. It is inorganic compound, food supplement E510, used as fertilizer, flavouring agent. Structural formula, molecule Stock Vectorhttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/ammonium-chloride-nh4cl-molecule-it-is-inorganic-compound-food-supplement-e510-used-as-fertilizer-flavouring-agent-structural-formula-molecule-image457383705.htmlRF2HG3HG9–Ammonium chloride, NH4Cl molecule. It is inorganic compound, food supplement E510, used as fertilizer, flavouring agent. Structural formula, molecule
 Salmiac pastilles in a closeup Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/salmiac-pastilles-in-a-closeup-image335687200.html
Salmiac pastilles in a closeup Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/salmiac-pastilles-in-a-closeup-image335687200.htmlRF2AE3TCG–Salmiac pastilles in a closeup
 Sal ammoniac. minerals. Europe; Italy; Sicily; Mt. Etna Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/sal-ammoniac-minerals-europe-italy-sicily-mt-etna-image472976662.html
Sal ammoniac. minerals. Europe; Italy; Sicily; Mt. Etna Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/sal-ammoniac-minerals-europe-italy-sicily-mt-etna-image472976662.htmlRM2JDDXFJ–Sal ammoniac. minerals. Europe; Italy; Sicily; Mt. Etna
 . A Reference handbook of the medical sciences : embracing the entire range of scientific and practical medicine and allied science. rly diluted hydro-chloric or sulphuric acid. The metal is then dipped introughs containing the spelter, melted zinc, and fromtime to time sal ammoniac is sprinkled on the surface ofthe bath for the purpose of cleaning the melted spelter byabsorption of the oxide. This viscous and inactive oxi-chloride of zinc is skimmed off. The galvanizers scum issubsequently treated for the recovery of the sal ammoniacand spelter. Galvanizing works are a source of nui-sance on Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/a-reference-handbook-of-the-medical-sciences-embracing-the-entire-range-of-scientific-and-practical-medicine-and-allied-science-rly-diluted-hydro-chloric-or-sulphuric-acid-the-metal-is-then-dipped-introughs-containing-the-spelter-melted-zinc-and-fromtime-to-time-sal-ammoniac-is-sprinkled-on-the-surface-ofthe-bath-for-the-purpose-of-cleaning-the-melted-spelter-byabsorption-of-the-oxide-this-viscous-and-inactive-oxi-chloride-of-zinc-is-skimmed-off-the-galvanizers-scum-issubsequently-treated-for-the-recovery-of-the-sal-ammoniacand-spelter-galvanizing-works-are-a-source-of-nui-sance-on-image337023151.html
. A Reference handbook of the medical sciences : embracing the entire range of scientific and practical medicine and allied science. rly diluted hydro-chloric or sulphuric acid. The metal is then dipped introughs containing the spelter, melted zinc, and fromtime to time sal ammoniac is sprinkled on the surface ofthe bath for the purpose of cleaning the melted spelter byabsorption of the oxide. This viscous and inactive oxi-chloride of zinc is skimmed off. The galvanizers scum issubsequently treated for the recovery of the sal ammoniacand spelter. Galvanizing works are a source of nui-sance on Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/a-reference-handbook-of-the-medical-sciences-embracing-the-entire-range-of-scientific-and-practical-medicine-and-allied-science-rly-diluted-hydro-chloric-or-sulphuric-acid-the-metal-is-then-dipped-introughs-containing-the-spelter-melted-zinc-and-fromtime-to-time-sal-ammoniac-is-sprinkled-on-the-surface-ofthe-bath-for-the-purpose-of-cleaning-the-melted-spelter-byabsorption-of-the-oxide-this-viscous-and-inactive-oxi-chloride-of-zinc-is-skimmed-off-the-galvanizers-scum-issubsequently-treated-for-the-recovery-of-the-sal-ammoniacand-spelter-galvanizing-works-are-a-source-of-nui-sance-on-image337023151.htmlRM2AG8MD3–. A Reference handbook of the medical sciences : embracing the entire range of scientific and practical medicine and allied science. rly diluted hydro-chloric or sulphuric acid. The metal is then dipped introughs containing the spelter, melted zinc, and fromtime to time sal ammoniac is sprinkled on the surface ofthe bath for the purpose of cleaning the melted spelter byabsorption of the oxide. This viscous and inactive oxi-chloride of zinc is skimmed off. The galvanizers scum issubsequently treated for the recovery of the sal ammoniacand spelter. Galvanizing works are a source of nui-sance on
 Salmiac pastilles in a closeup Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/salmiac-pastilles-in-a-closeup-image351455133.html
Salmiac pastilles in a closeup Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/salmiac-pastilles-in-a-closeup-image351455133.htmlRF2BBP4H1–Salmiac pastilles in a closeup
 Sal ammoniac. minerals. North America; Mexico; Paricutin Volcano Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/sal-ammoniac-minerals-north-america-mexico-paricutin-volcano-image472969038.html
Sal ammoniac. minerals. North America; Mexico; Paricutin Volcano Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/sal-ammoniac-minerals-north-america-mexico-paricutin-volcano-image472969038.htmlRM2JDDGRA–Sal ammoniac. minerals. North America; Mexico; Paricutin Volcano
 The diseases of sheep explained and described . ly con-sidered one of the most important remedies; at the presenttime, however, this remedy is not so frequently resorted to,on account of the employment of attenuating, cooling oraperient remedies, whose effects are preferable to those ofvenesection. The principal remedies employed in inflamma-tions are saltpetre, tartar, epsom, bitter or glauber salts; sal-ammoniac, sulphuric acid, vinegar or yeast diluted withwater, mixed with and served instead of the pure branamong the fodder or drink, or administered to the animalin form of a drink or injec Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/the-diseases-of-sheep-explained-and-described-ly-con-sidered-one-of-the-most-important-remedies-at-the-presenttime-however-this-remedy-is-not-so-frequently-resorted-toon-account-of-the-employment-of-attenuating-cooling-oraperient-remedies-whose-effects-are-preferable-to-those-ofvenesection-the-principal-remedies-employed-in-inflamma-tions-are-saltpetre-tartar-epsom-bitter-or-glauber-salts-sal-ammoniac-sulphuric-acid-vinegar-or-yeast-diluted-withwater-mixed-with-and-served-instead-of-the-pure-branamong-the-fodder-or-drink-or-administered-to-the-animalin-form-of-a-drink-or-injec-image338339382.html
The diseases of sheep explained and described . ly con-sidered one of the most important remedies; at the presenttime, however, this remedy is not so frequently resorted to,on account of the employment of attenuating, cooling oraperient remedies, whose effects are preferable to those ofvenesection. The principal remedies employed in inflamma-tions are saltpetre, tartar, epsom, bitter or glauber salts; sal-ammoniac, sulphuric acid, vinegar or yeast diluted withwater, mixed with and served instead of the pure branamong the fodder or drink, or administered to the animalin form of a drink or injec Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/the-diseases-of-sheep-explained-and-described-ly-con-sidered-one-of-the-most-important-remedies-at-the-presenttime-however-this-remedy-is-not-so-frequently-resorted-toon-account-of-the-employment-of-attenuating-cooling-oraperient-remedies-whose-effects-are-preferable-to-those-ofvenesection-the-principal-remedies-employed-in-inflamma-tions-are-saltpetre-tartar-epsom-bitter-or-glauber-salts-sal-ammoniac-sulphuric-acid-vinegar-or-yeast-diluted-withwater-mixed-with-and-served-instead-of-the-pure-branamong-the-fodder-or-drink-or-administered-to-the-animalin-form-of-a-drink-or-injec-image338339382.htmlRM2AJCK9A–The diseases of sheep explained and described . ly con-sidered one of the most important remedies; at the presenttime, however, this remedy is not so frequently resorted to,on account of the employment of attenuating, cooling oraperient remedies, whose effects are preferable to those ofvenesection. The principal remedies employed in inflamma-tions are saltpetre, tartar, epsom, bitter or glauber salts; sal-ammoniac, sulphuric acid, vinegar or yeast diluted withwater, mixed with and served instead of the pure branamong the fodder or drink, or administered to the animalin form of a drink or injec
 Sal ammoniac. minerals. Europe; Italy; Campania Region; Mt. Vesuvius Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/sal-ammoniac-minerals-europe-italy-campania-region-mt-vesuvius-image472970506.html
Sal ammoniac. minerals. Europe; Italy; Campania Region; Mt. Vesuvius Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/sal-ammoniac-minerals-europe-italy-campania-region-mt-vesuvius-image472970506.htmlRM2JDDJKP–Sal ammoniac. minerals. Europe; Italy; Campania Region; Mt. Vesuvius
 A supplement to Ures Dictionary of Arts, Manufactures, and Mines, : containing a clear exposition of their principles and practice. . AMMONIA, OAEBONATE OF. 85 Carbonate of ammonia is formed during the putrefaction of animal substances, and bytheir destructive distillation. Its presence in rain water has been before alluded to. The carbonate of ammonia of commerce is obtained by submitting to sublimation amixture either of sal ammoniac or sulphate of ammonia with chalk. This is generally carried out in cast-iron retorts, similar in size and shape to those usedin the manufacture of coal gas. Ti Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/a-supplement-to-ures-dictionary-of-arts-manufactures-and-mines-containing-a-clear-exposition-of-their-principles-and-practice-ammonia-oaebonate-of-85-carbonate-of-ammonia-is-formed-during-the-putrefaction-of-animal-substances-and-bytheir-destructive-distillation-its-presence-in-rain-water-has-been-before-alluded-to-the-carbonate-of-ammonia-of-commerce-is-obtained-by-submitting-to-sublimation-amixture-either-of-sal-ammoniac-or-sulphate-of-ammonia-with-chalk-this-is-generally-carried-out-in-cast-iron-retorts-similar-in-size-and-shape-to-those-usedin-the-manufacture-of-coal-gas-ti-image340019131.html
A supplement to Ures Dictionary of Arts, Manufactures, and Mines, : containing a clear exposition of their principles and practice. . AMMONIA, OAEBONATE OF. 85 Carbonate of ammonia is formed during the putrefaction of animal substances, and bytheir destructive distillation. Its presence in rain water has been before alluded to. The carbonate of ammonia of commerce is obtained by submitting to sublimation amixture either of sal ammoniac or sulphate of ammonia with chalk. This is generally carried out in cast-iron retorts, similar in size and shape to those usedin the manufacture of coal gas. Ti Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/a-supplement-to-ures-dictionary-of-arts-manufactures-and-mines-containing-a-clear-exposition-of-their-principles-and-practice-ammonia-oaebonate-of-85-carbonate-of-ammonia-is-formed-during-the-putrefaction-of-animal-substances-and-bytheir-destructive-distillation-its-presence-in-rain-water-has-been-before-alluded-to-the-carbonate-of-ammonia-of-commerce-is-obtained-by-submitting-to-sublimation-amixture-either-of-sal-ammoniac-or-sulphate-of-ammonia-with-chalk-this-is-generally-carried-out-in-cast-iron-retorts-similar-in-size-and-shape-to-those-usedin-the-manufacture-of-coal-gas-ti-image340019131.htmlRM2AN55TB–A supplement to Ures Dictionary of Arts, Manufactures, and Mines, : containing a clear exposition of their principles and practice. . AMMONIA, OAEBONATE OF. 85 Carbonate of ammonia is formed during the putrefaction of animal substances, and bytheir destructive distillation. Its presence in rain water has been before alluded to. The carbonate of ammonia of commerce is obtained by submitting to sublimation amixture either of sal ammoniac or sulphate of ammonia with chalk. This is generally carried out in cast-iron retorts, similar in size and shape to those usedin the manufacture of coal gas. Ti
 Sal ammoniac. minerals. Europe; Italy; Campania Region; Mt. Vesuvius Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/sal-ammoniac-minerals-europe-italy-campania-region-mt-vesuvius-image472965275.html
Sal ammoniac. minerals. Europe; Italy; Campania Region; Mt. Vesuvius Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/sal-ammoniac-minerals-europe-italy-campania-region-mt-vesuvius-image472965275.htmlRM2JDDC0Y–Sal ammoniac. minerals. Europe; Italy; Campania Region; Mt. Vesuvius
 Scientific amusements . named hypothetically AMMONIUM.It is usually classed amongstthe alkaline metals. The saltsof ammonia are important,and have already been men-tioned. Muriate (chloride) ofammonia, or sal-ammoniac, isanalogous to chloride ofsodium and chloride of potas-sium. It is decomposed byMottled soap-frames. heating it with slakcd lime and then gaseous ammonia is given off. The Metals of the Alkaline Earths. Barium is the first of the four metals we have to noticein this group, and will not detainus long, for it is little known in afree condition. Its most im-portant compound is heav Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/scientific-amusements-named-hypothetically-ammoniumit-is-usually-classed-amongstthe-alkaline-metals-the-saltsof-ammonia-are-importantand-have-already-been-men-tioned-muriate-chloride-ofammonia-or-sal-ammoniac-isanalogous-to-chloride-ofsodium-and-chloride-of-potas-sium-it-is-decomposed-bymottled-soap-frames-heating-it-with-slakcd-lime-and-then-gaseous-ammonia-is-given-off-the-metals-of-the-alkaline-earths-barium-is-the-first-of-the-four-metals-we-have-to-noticein-this-group-and-will-not-detainus-long-for-it-is-little-known-in-afree-condition-its-most-im-portant-compound-is-heav-image343302515.html
Scientific amusements . named hypothetically AMMONIUM.It is usually classed amongstthe alkaline metals. The saltsof ammonia are important,and have already been men-tioned. Muriate (chloride) ofammonia, or sal-ammoniac, isanalogous to chloride ofsodium and chloride of potas-sium. It is decomposed byMottled soap-frames. heating it with slakcd lime and then gaseous ammonia is given off. The Metals of the Alkaline Earths. Barium is the first of the four metals we have to noticein this group, and will not detainus long, for it is little known in afree condition. Its most im-portant compound is heav Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/scientific-amusements-named-hypothetically-ammoniumit-is-usually-classed-amongstthe-alkaline-metals-the-saltsof-ammonia-are-importantand-have-already-been-men-tioned-muriate-chloride-ofammonia-or-sal-ammoniac-isanalogous-to-chloride-ofsodium-and-chloride-of-potas-sium-it-is-decomposed-bymottled-soap-frames-heating-it-with-slakcd-lime-and-then-gaseous-ammonia-is-given-off-the-metals-of-the-alkaline-earths-barium-is-the-first-of-the-four-metals-we-have-to-noticein-this-group-and-will-not-detainus-long-for-it-is-little-known-in-afree-condition-its-most-im-portant-compound-is-heav-image343302515.htmlRM2AXENT3–Scientific amusements . named hypothetically AMMONIUM.It is usually classed amongstthe alkaline metals. The saltsof ammonia are important,and have already been men-tioned. Muriate (chloride) ofammonia, or sal-ammoniac, isanalogous to chloride ofsodium and chloride of potas-sium. It is decomposed byMottled soap-frames. heating it with slakcd lime and then gaseous ammonia is given off. The Metals of the Alkaline Earths. Barium is the first of the four metals we have to noticein this group, and will not detainus long, for it is little known in afree condition. Its most im-portant compound is heav
 Sal ammoniac. minerals. Europe; Italy; Campania Region; Mt. Vesuvius Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/sal-ammoniac-minerals-europe-italy-campania-region-mt-vesuvius-image472965807.html
Sal ammoniac. minerals. Europe; Italy; Campania Region; Mt. Vesuvius Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/sal-ammoniac-minerals-europe-italy-campania-region-mt-vesuvius-image472965807.htmlRM2JDDCKY–Sal ammoniac. minerals. Europe; Italy; Campania Region; Mt. Vesuvius
 The elements of materia medica and therapeutics . Coals.—In the manufacture ofcoal gas, coal is submitted to distillation in iron retorts, and the vola-tile matters obtained are conveyed to a condensing vessel or refrige-ratory, in which are deposited tar and an ammoniacal liquor. This ammoniacal liquor (commonly termed gas liquor) containsseveral salts of ammonia—such as carbonate, hydrocyanate, sulphate,and hydrosulphate. It is usually sold to sal-ammoniac manufac-turers, who reside in the outskirts of the metropolis. The precisemode of proceeding, to convert it into sal ammoniac, varies acc Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/the-elements-of-materia-medica-and-therapeutics-coalsin-the-manufacture-ofcoal-gas-coal-is-submitted-to-distillation-in-iron-retorts-and-the-vola-tile-matters-obtained-are-conveyed-to-a-condensing-vessel-or-refrige-ratory-in-which-are-deposited-tar-and-an-ammoniacal-liquor-this-ammoniacal-liquor-commonly-termed-gas-liquor-containsseveral-salts-of-ammoniasuch-as-carbonate-hydrocyanate-sulphateand-hydrosulphate-it-is-usually-sold-to-sal-ammoniac-manufac-turers-who-reside-in-the-outskirts-of-the-metropolis-the-precisemode-of-proceeding-to-convert-it-into-sal-ammoniac-varies-acc-image339123428.html
The elements of materia medica and therapeutics . Coals.—In the manufacture ofcoal gas, coal is submitted to distillation in iron retorts, and the vola-tile matters obtained are conveyed to a condensing vessel or refrige-ratory, in which are deposited tar and an ammoniacal liquor. This ammoniacal liquor (commonly termed gas liquor) containsseveral salts of ammonia—such as carbonate, hydrocyanate, sulphate,and hydrosulphate. It is usually sold to sal-ammoniac manufac-turers, who reside in the outskirts of the metropolis. The precisemode of proceeding, to convert it into sal ammoniac, varies acc Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/the-elements-of-materia-medica-and-therapeutics-coalsin-the-manufacture-ofcoal-gas-coal-is-submitted-to-distillation-in-iron-retorts-and-the-vola-tile-matters-obtained-are-conveyed-to-a-condensing-vessel-or-refrige-ratory-in-which-are-deposited-tar-and-an-ammoniacal-liquor-this-ammoniacal-liquor-commonly-termed-gas-liquor-containsseveral-salts-of-ammoniasuch-as-carbonate-hydrocyanate-sulphateand-hydrosulphate-it-is-usually-sold-to-sal-ammoniac-manufac-turers-who-reside-in-the-outskirts-of-the-metropolis-the-precisemode-of-proceeding-to-convert-it-into-sal-ammoniac-varies-acc-image339123428.htmlRM2AKMBB0–The elements of materia medica and therapeutics . Coals.—In the manufacture ofcoal gas, coal is submitted to distillation in iron retorts, and the vola-tile matters obtained are conveyed to a condensing vessel or refrige-ratory, in which are deposited tar and an ammoniacal liquor. This ammoniacal liquor (commonly termed gas liquor) containsseveral salts of ammonia—such as carbonate, hydrocyanate, sulphate,and hydrosulphate. It is usually sold to sal-ammoniac manufac-turers, who reside in the outskirts of the metropolis. The precisemode of proceeding, to convert it into sal ammoniac, varies acc
 Sal ammoniac. minerals. Europe; Italy; Campania Region; Mt. Vesuvius Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/sal-ammoniac-minerals-europe-italy-campania-region-mt-vesuvius-image472963378.html
Sal ammoniac. minerals. Europe; Italy; Campania Region; Mt. Vesuvius Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/sal-ammoniac-minerals-europe-italy-campania-region-mt-vesuvius-image472963378.htmlRM2JDD9H6–Sal ammoniac. minerals. Europe; Italy; Campania Region; Mt. Vesuvius
 Lessons in practical electricity; principles, experiments, and arithmetical problems, an elementary text-book . Fig. 87.—GondaLeclancheElements.. Fig. 88.—Elements of a Carbon Cylinder Cell.Zinc, carbon and sal-ammoniac solution. BATTERIES. 73 carbon is madewith oxide of cell of the Standard Carbon Co., in which the in the form of a porous cup and then fillec manganese to prevent polarization. Still another form of the same make is shown in Fig. 90, in which the space between the two concentric carbon cylinders has been filled with oxide of Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/lessons-in-practical-electricity-principles-experiments-and-arithmetical-problems-an-elementary-text-book-fig-87gondaleclancheelements-fig-88elements-of-a-carbon-cylinder-cellzinc-carbon-and-sal-ammoniac-solution-batteries-73-carbon-is-madewith-oxide-of-cell-of-the-standard-carbon-co-in-which-the-in-the-form-of-a-porous-cup-and-then-fillec-manganese-to-prevent-polarization-still-another-form-of-the-same-make-is-shown-in-fig-90-in-which-the-space-between-the-two-concentric-carbon-cylinders-has-been-filled-with-oxide-of-image340072663.html
Lessons in practical electricity; principles, experiments, and arithmetical problems, an elementary text-book . Fig. 87.—GondaLeclancheElements.. Fig. 88.—Elements of a Carbon Cylinder Cell.Zinc, carbon and sal-ammoniac solution. BATTERIES. 73 carbon is madewith oxide of cell of the Standard Carbon Co., in which the in the form of a porous cup and then fillec manganese to prevent polarization. Still another form of the same make is shown in Fig. 90, in which the space between the two concentric carbon cylinders has been filled with oxide of Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/lessons-in-practical-electricity-principles-experiments-and-arithmetical-problems-an-elementary-text-book-fig-87gondaleclancheelements-fig-88elements-of-a-carbon-cylinder-cellzinc-carbon-and-sal-ammoniac-solution-batteries-73-carbon-is-madewith-oxide-of-cell-of-the-standard-carbon-co-in-which-the-in-the-form-of-a-porous-cup-and-then-fillec-manganese-to-prevent-polarization-still-another-form-of-the-same-make-is-shown-in-fig-90-in-which-the-space-between-the-two-concentric-carbon-cylinders-has-been-filled-with-oxide-of-image340072663.htmlRM2AN7J47–Lessons in practical electricity; principles, experiments, and arithmetical problems, an elementary text-book . Fig. 87.—GondaLeclancheElements.. Fig. 88.—Elements of a Carbon Cylinder Cell.Zinc, carbon and sal-ammoniac solution. BATTERIES. 73 carbon is madewith oxide of cell of the Standard Carbon Co., in which the in the form of a porous cup and then fillec manganese to prevent polarization. Still another form of the same make is shown in Fig. 90, in which the space between the two concentric carbon cylinders has been filled with oxide of
 Sal ammoniac. minerals. Europe; Italy; Campania Region; Mt. Vesuvius Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/sal-ammoniac-minerals-europe-italy-campania-region-mt-vesuvius-image472967914.html
Sal ammoniac. minerals. Europe; Italy; Campania Region; Mt. Vesuvius Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/sal-ammoniac-minerals-europe-italy-campania-region-mt-vesuvius-image472967914.htmlRM2JDDFB6–Sal ammoniac. minerals. Europe; Italy; Campania Region; Mt. Vesuvius
 Lessons in practical electricity; principles, experiments, and arithmetical problems, an elementary text-book . evaporates. The cell is then ready for use. 89. Gonda Leclanche Cell.—This cell is a modificationof the porous cup type, in which the manganese has beenmixed with some gelatinous binder, andcompressed into slabs, under hydraulicpressure. Two such slabs or prisms, oneon each side of the carbon plate, are held inposition by rubber bands, Fig. 87. A zincrod and a sal-ammoniac solution are used.This cell was designed to dispense with theporous cup. 90. Carbon Cylinder Cell.—Carbonpossess Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/lessons-in-practical-electricity-principles-experiments-and-arithmetical-problems-an-elementary-text-book-evaporates-the-cell-is-then-ready-for-use-89-gonda-leclanche-cellthis-cell-is-a-modificationof-the-porous-cup-type-in-which-the-manganese-has-beenmixed-with-some-gelatinous-binder-andcompressed-into-slabs-under-hydraulicpressure-two-such-slabs-or-prisms-oneon-each-side-of-the-carbon-plate-are-held-inposition-by-rubber-bands-fig-87-a-zincrod-and-a-sal-ammoniac-solution-are-usedthis-cell-was-designed-to-dispense-with-theporous-cup-90-carbon-cylinder-cellcarbonpossess-image340073179.html
Lessons in practical electricity; principles, experiments, and arithmetical problems, an elementary text-book . evaporates. The cell is then ready for use. 89. Gonda Leclanche Cell.—This cell is a modificationof the porous cup type, in which the manganese has beenmixed with some gelatinous binder, andcompressed into slabs, under hydraulicpressure. Two such slabs or prisms, oneon each side of the carbon plate, are held inposition by rubber bands, Fig. 87. A zincrod and a sal-ammoniac solution are used.This cell was designed to dispense with theporous cup. 90. Carbon Cylinder Cell.—Carbonpossess Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/lessons-in-practical-electricity-principles-experiments-and-arithmetical-problems-an-elementary-text-book-evaporates-the-cell-is-then-ready-for-use-89-gonda-leclanche-cellthis-cell-is-a-modificationof-the-porous-cup-type-in-which-the-manganese-has-beenmixed-with-some-gelatinous-binder-andcompressed-into-slabs-under-hydraulicpressure-two-such-slabs-or-prisms-oneon-each-side-of-the-carbon-plate-are-held-inposition-by-rubber-bands-fig-87-a-zincrod-and-a-sal-ammoniac-solution-are-usedthis-cell-was-designed-to-dispense-with-theporous-cup-90-carbon-cylinder-cellcarbonpossess-image340073179.htmlRM2AN7JPK–Lessons in practical electricity; principles, experiments, and arithmetical problems, an elementary text-book . evaporates. The cell is then ready for use. 89. Gonda Leclanche Cell.—This cell is a modificationof the porous cup type, in which the manganese has beenmixed with some gelatinous binder, andcompressed into slabs, under hydraulicpressure. Two such slabs or prisms, oneon each side of the carbon plate, are held inposition by rubber bands, Fig. 87. A zincrod and a sal-ammoniac solution are used.This cell was designed to dispense with theporous cup. 90. Carbon Cylinder Cell.—Carbonpossess
 Sal ammoniac. minerals. Europe; Italy; Campania Region; Mt. Vesuvius Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/sal-ammoniac-minerals-europe-italy-campania-region-mt-vesuvius-image472965782.html
Sal ammoniac. minerals. Europe; Italy; Campania Region; Mt. Vesuvius Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/sal-ammoniac-minerals-europe-italy-campania-region-mt-vesuvius-image472965782.htmlRM2JDDCK2–Sal ammoniac. minerals. Europe; Italy; Campania Region; Mt. Vesuvius
 A text-book on chemistry : for the use of schools and colleges . the air is dryer, the cool-ing must be carried to a greater extent. The precisethermometric point at which the moisture begins to de-posit is called the dew point. Thus, if we take a thin metallic vessel containing water,and cool it gradually by the addition of a mixture ofnitrate of potash and sal ammoniac, or any of the coolingmixtures, continually stirring with the bulb of a smallthermometer, as soon as the temperature has reached aF>g. 38. certain point a dew is deposited on the outsideof the metallic vessel ;that temperat Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/a-text-book-on-chemistry-for-the-use-of-schools-and-colleges-the-air-is-dryer-the-cool-ing-must-be-carried-to-a-greater-extent-the-precisethermometric-point-at-which-the-moisture-begins-to-de-posit-is-called-the-dew-point-thus-if-we-take-a-thin-metallic-vessel-containing-waterand-cool-it-gradually-by-the-addition-of-a-mixture-ofnitrate-of-potash-and-sal-ammoniac-or-any-of-the-coolingmixtures-continually-stirring-with-the-bulb-of-a-smallthermometer-as-soon-as-the-temperature-has-reached-afgtg-38-certain-point-a-dew-is-deposited-on-the-outsideof-the-metallic-vessel-that-temperat-image339369093.html
A text-book on chemistry : for the use of schools and colleges . the air is dryer, the cool-ing must be carried to a greater extent. The precisethermometric point at which the moisture begins to de-posit is called the dew point. Thus, if we take a thin metallic vessel containing water,and cool it gradually by the addition of a mixture ofnitrate of potash and sal ammoniac, or any of the coolingmixtures, continually stirring with the bulb of a smallthermometer, as soon as the temperature has reached aF>g. 38. certain point a dew is deposited on the outsideof the metallic vessel ;that temperat Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/a-text-book-on-chemistry-for-the-use-of-schools-and-colleges-the-air-is-dryer-the-cool-ing-must-be-carried-to-a-greater-extent-the-precisethermometric-point-at-which-the-moisture-begins-to-de-posit-is-called-the-dew-point-thus-if-we-take-a-thin-metallic-vessel-containing-waterand-cool-it-gradually-by-the-addition-of-a-mixture-ofnitrate-of-potash-and-sal-ammoniac-or-any-of-the-coolingmixtures-continually-stirring-with-the-bulb-of-a-smallthermometer-as-soon-as-the-temperature-has-reached-afgtg-38-certain-point-a-dew-is-deposited-on-the-outsideof-the-metallic-vessel-that-temperat-image339369093.htmlRM2AM3GMN–A text-book on chemistry : for the use of schools and colleges . the air is dryer, the cool-ing must be carried to a greater extent. The precisethermometric point at which the moisture begins to de-posit is called the dew point. Thus, if we take a thin metallic vessel containing water,and cool it gradually by the addition of a mixture ofnitrate of potash and sal ammoniac, or any of the coolingmixtures, continually stirring with the bulb of a smallthermometer, as soon as the temperature has reached aF>g. 38. certain point a dew is deposited on the outsideof the metallic vessel ;that temperat
 Sal ammoniac. minerals. Europe; Italy; Campania Region; Mt. Vesuvius Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/sal-ammoniac-minerals-europe-italy-campania-region-mt-vesuvius-image472972036.html
Sal ammoniac. minerals. Europe; Italy; Campania Region; Mt. Vesuvius Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/sal-ammoniac-minerals-europe-italy-campania-region-mt-vesuvius-image472972036.htmlRM2JDDMJC–Sal ammoniac. minerals. Europe; Italy; Campania Region; Mt. Vesuvius
 An elementary book on electricity and magnetism and their applications . of pulp board or blottingpaper which is saturated with asolution of sal ammoniac and zincchloride. The zinc chloride isnecessary to reduce the rapid de-terioration which would otherwisetake place on open circuit. Thespace between the lining and the carbon electrode is filled witha mixture of granulated carbon and manganese dioxide. Thislatter is used as the depolarizer. The top of the cell is gener-ally sealed up with a pitch composition. This cell has a volt-age of about 1.5 volts and will polarize rapidly if kept long o Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/an-elementary-book-on-electricity-and-magnetism-and-their-applications-of-pulp-board-or-blottingpaper-which-is-saturated-with-asolution-of-sal-ammoniac-and-zincchloride-the-zinc-chloride-isnecessary-to-reduce-the-rapid-de-terioration-which-would-otherwisetake-place-on-open-circuit-thespace-between-the-lining-and-the-carbon-electrode-is-filled-witha-mixture-of-granulated-carbon-and-manganese-dioxide-thislatter-is-used-as-the-depolarizer-the-top-of-the-cell-is-gener-ally-sealed-up-with-a-pitch-composition-this-cell-has-a-volt-age-of-about-15-volts-and-will-polarize-rapidly-if-kept-long-o-image338122341.html
An elementary book on electricity and magnetism and their applications . of pulp board or blottingpaper which is saturated with asolution of sal ammoniac and zincchloride. The zinc chloride isnecessary to reduce the rapid de-terioration which would otherwisetake place on open circuit. Thespace between the lining and the carbon electrode is filled witha mixture of granulated carbon and manganese dioxide. Thislatter is used as the depolarizer. The top of the cell is gener-ally sealed up with a pitch composition. This cell has a volt-age of about 1.5 volts and will polarize rapidly if kept long o Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/an-elementary-book-on-electricity-and-magnetism-and-their-applications-of-pulp-board-or-blottingpaper-which-is-saturated-with-asolution-of-sal-ammoniac-and-zincchloride-the-zinc-chloride-isnecessary-to-reduce-the-rapid-de-terioration-which-would-otherwisetake-place-on-open-circuit-thespace-between-the-lining-and-the-carbon-electrode-is-filled-witha-mixture-of-granulated-carbon-and-manganese-dioxide-thislatter-is-used-as-the-depolarizer-the-top-of-the-cell-is-gener-ally-sealed-up-with-a-pitch-composition-this-cell-has-a-volt-age-of-about-15-volts-and-will-polarize-rapidly-if-kept-long-o-image338122341.htmlRM2AJ2PDW–An elementary book on electricity and magnetism and their applications . of pulp board or blottingpaper which is saturated with asolution of sal ammoniac and zincchloride. The zinc chloride isnecessary to reduce the rapid de-terioration which would otherwisetake place on open circuit. Thespace between the lining and the carbon electrode is filled witha mixture of granulated carbon and manganese dioxide. Thislatter is used as the depolarizer. The top of the cell is gener-ally sealed up with a pitch composition. This cell has a volt-age of about 1.5 volts and will polarize rapidly if kept long o
 Sal ammoniac. minerals. Europe; Italy; Campania Region; Mt. Vesuvius Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/sal-ammoniac-minerals-europe-italy-campania-region-mt-vesuvius-image472978726.html
Sal ammoniac. minerals. Europe; Italy; Campania Region; Mt. Vesuvius Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/sal-ammoniac-minerals-europe-italy-campania-region-mt-vesuvius-image472978726.htmlRM2JDE15A–Sal ammoniac. minerals. Europe; Italy; Campania Region; Mt. Vesuvius
 . Medical electricity; a practical treatise on the applications of electricity to medicine and surgery. rs. Theelements are composed of zinc and gas carbon, the latterplaced in a porous cell and surrounded with native per-oxide of manganese, mixed with coarsely powdered carbon.The porous cell with its contents is placed in a glass vesselof quadrangular shape containing a saturated solution ofammonium chloride (sal ammoniac) and a rod of zinc.Ammonia is set free and absorbed by the water, chlorideof zinc is formed, and hydrogen is set free, but its polariza-tion is prevented by combination with Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/medical-electricity-a-practical-treatise-on-the-applications-of-electricity-to-medicine-and-surgery-rs-theelements-are-composed-of-zinc-and-gas-carbon-the-latterplaced-in-a-porous-cell-and-surrounded-with-native-per-oxide-of-manganese-mixed-with-coarsely-powdered-carbonthe-porous-cell-with-its-contents-is-placed-in-a-glass-vesselof-quadrangular-shape-containing-a-saturated-solution-ofammonium-chloride-sal-ammoniac-and-a-rod-of-zincammonia-is-set-free-and-absorbed-by-the-water-chlorideof-zinc-is-formed-and-hydrogen-is-set-free-but-its-polariza-tion-is-prevented-by-combination-with-image336949893.html
. Medical electricity; a practical treatise on the applications of electricity to medicine and surgery. rs. Theelements are composed of zinc and gas carbon, the latterplaced in a porous cell and surrounded with native per-oxide of manganese, mixed with coarsely powdered carbon.The porous cell with its contents is placed in a glass vesselof quadrangular shape containing a saturated solution ofammonium chloride (sal ammoniac) and a rod of zinc.Ammonia is set free and absorbed by the water, chlorideof zinc is formed, and hydrogen is set free, but its polariza-tion is prevented by combination with Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/medical-electricity-a-practical-treatise-on-the-applications-of-electricity-to-medicine-and-surgery-rs-theelements-are-composed-of-zinc-and-gas-carbon-the-latterplaced-in-a-porous-cell-and-surrounded-with-native-per-oxide-of-manganese-mixed-with-coarsely-powdered-carbonthe-porous-cell-with-its-contents-is-placed-in-a-glass-vesselof-quadrangular-shape-containing-a-saturated-solution-ofammonium-chloride-sal-ammoniac-and-a-rod-of-zincammonia-is-set-free-and-absorbed-by-the-water-chlorideof-zinc-is-formed-and-hydrogen-is-set-free-but-its-polariza-tion-is-prevented-by-combination-with-image336949893.htmlRM2AG5B0N–. Medical electricity; a practical treatise on the applications of electricity to medicine and surgery. rs. Theelements are composed of zinc and gas carbon, the latterplaced in a porous cell and surrounded with native per-oxide of manganese, mixed with coarsely powdered carbon.The porous cell with its contents is placed in a glass vesselof quadrangular shape containing a saturated solution ofammonium chloride (sal ammoniac) and a rod of zinc.Ammonia is set free and absorbed by the water, chlorideof zinc is formed, and hydrogen is set free, but its polariza-tion is prevented by combination with
 Sal ammoniac. minerals. Europe; Italy; Campania Region; Mt. Vesuvius Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/sal-ammoniac-minerals-europe-italy-campania-region-mt-vesuvius-image472965460.html
Sal ammoniac. minerals. Europe; Italy; Campania Region; Mt. Vesuvius Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/sal-ammoniac-minerals-europe-italy-campania-region-mt-vesuvius-image472965460.htmlRM2JDDC7G–Sal ammoniac. minerals. Europe; Italy; Campania Region; Mt. Vesuvius
![St Nicholas [serial] . carbon is used in place ofthe copper, a solution of sal ammoniac may beused instead of the sulphuric acid. The sulphuricacid should be used with great care. When notdiluted, it will injure flesh or clothing with whichit conies in contact. The crowfoot battery (Fig. 2), so calledfrom the shape of the zinc which is suspendedfrom the top of the jar, with the solution justcovering it, is almost exactly the same kind ofbattery as the one made with the tumbler and thestrips of zinc and copper. The copper of thisbattery you will see spread out on the bottom ofthe jar. The small Stock Photo St Nicholas [serial] . carbon is used in place ofthe copper, a solution of sal ammoniac may beused instead of the sulphuric acid. The sulphuricacid should be used with great care. When notdiluted, it will injure flesh or clothing with whichit conies in contact. The crowfoot battery (Fig. 2), so calledfrom the shape of the zinc which is suspendedfrom the top of the jar, with the solution justcovering it, is almost exactly the same kind ofbattery as the one made with the tumbler and thestrips of zinc and copper. The copper of thisbattery you will see spread out on the bottom ofthe jar. The small Stock Photo](https://c8.alamy.com/comp/2AJ8HRR/st-nicholas-serial-carbon-is-used-in-place-ofthe-copper-a-solution-of-sal-ammoniac-may-beused-instead-of-the-sulphuric-acid-the-sulphuricacid-should-be-used-with-great-care-when-notdiluted-it-will-injure-flesh-or-clothing-with-whichit-conies-in-contact-the-crowfoot-battery-fig-2-so-calledfrom-the-shape-of-the-zinc-which-is-suspendedfrom-the-top-of-the-jar-with-the-solution-justcovering-it-is-almost-exactly-the-same-kind-ofbattery-as-the-one-made-with-the-tumbler-and-thestrips-of-zinc-and-copper-the-copper-of-thisbattery-you-will-see-spread-out-on-the-bottom-ofthe-jar-the-small-2AJ8HRR.jpg) St Nicholas [serial] . carbon is used in place ofthe copper, a solution of sal ammoniac may beused instead of the sulphuric acid. The sulphuricacid should be used with great care. When notdiluted, it will injure flesh or clothing with whichit conies in contact. The crowfoot battery (Fig. 2), so calledfrom the shape of the zinc which is suspendedfrom the top of the jar, with the solution justcovering it, is almost exactly the same kind ofbattery as the one made with the tumbler and thestrips of zinc and copper. The copper of thisbattery you will see spread out on the bottom ofthe jar. The small Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/st-nicholas-serial-carbon-is-used-in-place-ofthe-copper-a-solution-of-sal-ammoniac-may-beused-instead-of-the-sulphuric-acid-the-sulphuricacid-should-be-used-with-great-care-when-notdiluted-it-will-injure-flesh-or-clothing-with-whichit-conies-in-contact-the-crowfoot-battery-fig-2-so-calledfrom-the-shape-of-the-zinc-which-is-suspendedfrom-the-top-of-the-jar-with-the-solution-justcovering-it-is-almost-exactly-the-same-kind-ofbattery-as-the-one-made-with-the-tumbler-and-thestrips-of-zinc-and-copper-the-copper-of-thisbattery-you-will-see-spread-out-on-the-bottom-ofthe-jar-the-small-image338250411.html
St Nicholas [serial] . carbon is used in place ofthe copper, a solution of sal ammoniac may beused instead of the sulphuric acid. The sulphuricacid should be used with great care. When notdiluted, it will injure flesh or clothing with whichit conies in contact. The crowfoot battery (Fig. 2), so calledfrom the shape of the zinc which is suspendedfrom the top of the jar, with the solution justcovering it, is almost exactly the same kind ofbattery as the one made with the tumbler and thestrips of zinc and copper. The copper of thisbattery you will see spread out on the bottom ofthe jar. The small Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/st-nicholas-serial-carbon-is-used-in-place-ofthe-copper-a-solution-of-sal-ammoniac-may-beused-instead-of-the-sulphuric-acid-the-sulphuricacid-should-be-used-with-great-care-when-notdiluted-it-will-injure-flesh-or-clothing-with-whichit-conies-in-contact-the-crowfoot-battery-fig-2-so-calledfrom-the-shape-of-the-zinc-which-is-suspendedfrom-the-top-of-the-jar-with-the-solution-justcovering-it-is-almost-exactly-the-same-kind-ofbattery-as-the-one-made-with-the-tumbler-and-thestrips-of-zinc-and-copper-the-copper-of-thisbattery-you-will-see-spread-out-on-the-bottom-ofthe-jar-the-small-image338250411.htmlRM2AJ8HRR–St Nicholas [serial] . carbon is used in place ofthe copper, a solution of sal ammoniac may beused instead of the sulphuric acid. The sulphuricacid should be used with great care. When notdiluted, it will injure flesh or clothing with whichit conies in contact. The crowfoot battery (Fig. 2), so calledfrom the shape of the zinc which is suspendedfrom the top of the jar, with the solution justcovering it, is almost exactly the same kind ofbattery as the one made with the tumbler and thestrips of zinc and copper. The copper of thisbattery you will see spread out on the bottom ofthe jar. The small
 Cooley's cyclopaedia of practical receipts and collateral information in the arts, manufactures, professions, and trades including medicine, pharmacy, hygiene, and domestic economy : designed as a comprehensive supplement to the Pharmacopoeia and general book of reference for the manufacturer, tradesman, amateur, and heads of families . Prepared by distilling, in a tubular retort,equal parts of sal ammoniac, hydrated lime,or slaiied lime and water, and passing the gas evolved thi-ougli a set of Wolffs bottlespartially filled with water, as in the figvu-oabove.. A, CyliDdrieal Iron Retort. B, F Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/cooleys-cyclopaedia-of-practical-receipts-and-collateral-information-in-the-arts-manufactures-professions-and-trades-including-medicine-pharmacy-hygiene-and-domestic-economy-designed-as-a-comprehensive-supplement-to-the-pharmacopoeia-and-general-book-of-reference-for-the-manufacturer-tradesman-amateur-and-heads-of-families-prepared-by-distilling-in-a-tubular-retortequal-parts-of-sal-ammoniac-hydrated-limeor-slaiied-lime-and-water-and-passing-the-gas-evolved-thi-ougli-a-set-of-wolffs-bottlespartially-filled-with-water-as-in-the-figvu-oabove-a-cyliddrieal-iron-retort-b-f-image340035491.html
Cooley's cyclopaedia of practical receipts and collateral information in the arts, manufactures, professions, and trades including medicine, pharmacy, hygiene, and domestic economy : designed as a comprehensive supplement to the Pharmacopoeia and general book of reference for the manufacturer, tradesman, amateur, and heads of families . Prepared by distilling, in a tubular retort,equal parts of sal ammoniac, hydrated lime,or slaiied lime and water, and passing the gas evolved thi-ougli a set of Wolffs bottlespartially filled with water, as in the figvu-oabove.. A, CyliDdrieal Iron Retort. B, F Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/cooleys-cyclopaedia-of-practical-receipts-and-collateral-information-in-the-arts-manufactures-professions-and-trades-including-medicine-pharmacy-hygiene-and-domestic-economy-designed-as-a-comprehensive-supplement-to-the-pharmacopoeia-and-general-book-of-reference-for-the-manufacturer-tradesman-amateur-and-heads-of-families-prepared-by-distilling-in-a-tubular-retortequal-parts-of-sal-ammoniac-hydrated-limeor-slaiied-lime-and-water-and-passing-the-gas-evolved-thi-ougli-a-set-of-wolffs-bottlespartially-filled-with-water-as-in-the-figvu-oabove-a-cyliddrieal-iron-retort-b-f-image340035491.htmlRM2AN5XMK–Cooley's cyclopaedia of practical receipts and collateral information in the arts, manufactures, professions, and trades including medicine, pharmacy, hygiene, and domestic economy : designed as a comprehensive supplement to the Pharmacopoeia and general book of reference for the manufacturer, tradesman, amateur, and heads of families . Prepared by distilling, in a tubular retort,equal parts of sal ammoniac, hydrated lime,or slaiied lime and water, and passing the gas evolved thi-ougli a set of Wolffs bottlespartially filled with water, as in the figvu-oabove.. A, CyliDdrieal Iron Retort. B, F
 . The American encyclopædia of commerce, manufactures, commercial law, and finance. liarprocess, whereby it resists the rusting influence ofdamp air, and even moisture, much longer thanordinary tin plate. It is made in corrugateilsheets, and ranges from 800 sq. feet per ton, to2,170 feet or more. It is cither curved, step-cor-rugated, or corrugated with small flutes or chan-nels. Mantif. Clean the surface of the iron perfectly by the jointaction of dilute acid and friction, plunge it into a bath of meltedzinc, covered with sal-ammoniac, and stir it about till it bealloyed superficially with th Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/the-american-encyclopdia-of-commerce-manufactures-commercial-law-and-finance-liarprocess-whereby-it-resists-the-rusting-influence-ofdamp-air-and-even-moisture-much-longer-thanordinary-tin-plate-it-is-made-in-corrugateilsheets-and-ranges-from-800-sq-feet-per-ton-to2170-feet-or-more-it-is-cither-curved-step-cor-rugated-or-corrugated-with-small-flutes-or-chan-nels-mantif-clean-the-surface-of-the-iron-perfectly-by-the-jointaction-of-dilute-acid-and-friction-plunge-it-into-a-bath-of-meltedzinc-covered-with-sal-ammoniac-and-stir-it-about-till-it-bealloyed-superficially-with-th-image336945635.html
. The American encyclopædia of commerce, manufactures, commercial law, and finance. liarprocess, whereby it resists the rusting influence ofdamp air, and even moisture, much longer thanordinary tin plate. It is made in corrugateilsheets, and ranges from 800 sq. feet per ton, to2,170 feet or more. It is cither curved, step-cor-rugated, or corrugated with small flutes or chan-nels. Mantif. Clean the surface of the iron perfectly by the jointaction of dilute acid and friction, plunge it into a bath of meltedzinc, covered with sal-ammoniac, and stir it about till it bealloyed superficially with th Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/the-american-encyclopdia-of-commerce-manufactures-commercial-law-and-finance-liarprocess-whereby-it-resists-the-rusting-influence-ofdamp-air-and-even-moisture-much-longer-thanordinary-tin-plate-it-is-made-in-corrugateilsheets-and-ranges-from-800-sq-feet-per-ton-to2170-feet-or-more-it-is-cither-curved-step-cor-rugated-or-corrugated-with-small-flutes-or-chan-nels-mantif-clean-the-surface-of-the-iron-perfectly-by-the-jointaction-of-dilute-acid-and-friction-plunge-it-into-a-bath-of-meltedzinc-covered-with-sal-ammoniac-and-stir-it-about-till-it-bealloyed-superficially-with-th-image336945635.htmlRM2AG55GK–. The American encyclopædia of commerce, manufactures, commercial law, and finance. liarprocess, whereby it resists the rusting influence ofdamp air, and even moisture, much longer thanordinary tin plate. It is made in corrugateilsheets, and ranges from 800 sq. feet per ton, to2,170 feet or more. It is cither curved, step-cor-rugated, or corrugated with small flutes or chan-nels. Mantif. Clean the surface of the iron perfectly by the jointaction of dilute acid and friction, plunge it into a bath of meltedzinc, covered with sal-ammoniac, and stir it about till it bealloyed superficially with th
![St Nicholas [serial] . shell is the zinc plate, to which issoldered a binding-post to hold the wire. Next,inside of this, is a porous paper lining for insula-tion. Extending down into this is a solid carboncylinder—any shape will do —surrounded bypowdered carbon wet with sal-ammoniac solu-tion. Sawdust would do in place of the carbonpowder, but would not be quite so good. Thepitch is poured into the top of the battery to pre-vent the evaporation of the moisture. These bat-teries come in a paper box, so they can be placedtogether, but do not let the metal parts come incontact, which would soon Stock Photo St Nicholas [serial] . shell is the zinc plate, to which issoldered a binding-post to hold the wire. Next,inside of this, is a porous paper lining for insula-tion. Extending down into this is a solid carboncylinder—any shape will do —surrounded bypowdered carbon wet with sal-ammoniac solu-tion. Sawdust would do in place of the carbonpowder, but would not be quite so good. Thepitch is poured into the top of the battery to pre-vent the evaporation of the moisture. These bat-teries come in a paper box, so they can be placedtogether, but do not let the metal parts come incontact, which would soon Stock Photo](https://c8.alamy.com/comp/2AJ8H45/st-nicholas-serial-shell-is-the-zinc-plate-to-which-issoldered-a-binding-post-to-hold-the-wire-nextinside-of-this-is-a-porous-paper-lining-for-insula-tion-extending-down-into-this-is-a-solid-carboncylinderany-shape-will-do-surrounded-bypowdered-carbon-wet-with-sal-ammoniac-solu-tion-sawdust-would-do-in-place-of-the-carbonpowder-but-would-not-be-quite-so-good-thepitch-is-poured-into-the-top-of-the-battery-to-pre-vent-the-evaporation-of-the-moisture-these-bat-teries-come-in-a-paper-box-so-they-can-be-placedtogether-but-do-not-let-the-metal-parts-come-incontact-which-would-soon-2AJ8H45.jpg) St Nicholas [serial] . shell is the zinc plate, to which issoldered a binding-post to hold the wire. Next,inside of this, is a porous paper lining for insula-tion. Extending down into this is a solid carboncylinder—any shape will do —surrounded bypowdered carbon wet with sal-ammoniac solu-tion. Sawdust would do in place of the carbonpowder, but would not be quite so good. Thepitch is poured into the top of the battery to pre-vent the evaporation of the moisture. These bat-teries come in a paper box, so they can be placedtogether, but do not let the metal parts come incontact, which would soon Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/st-nicholas-serial-shell-is-the-zinc-plate-to-which-issoldered-a-binding-post-to-hold-the-wire-nextinside-of-this-is-a-porous-paper-lining-for-insula-tion-extending-down-into-this-is-a-solid-carboncylinderany-shape-will-do-surrounded-bypowdered-carbon-wet-with-sal-ammoniac-solu-tion-sawdust-would-do-in-place-of-the-carbonpowder-but-would-not-be-quite-so-good-thepitch-is-poured-into-the-top-of-the-battery-to-pre-vent-the-evaporation-of-the-moisture-these-bat-teries-come-in-a-paper-box-so-they-can-be-placedtogether-but-do-not-let-the-metal-parts-come-incontact-which-would-soon-image338249861.html
St Nicholas [serial] . shell is the zinc plate, to which issoldered a binding-post to hold the wire. Next,inside of this, is a porous paper lining for insula-tion. Extending down into this is a solid carboncylinder—any shape will do —surrounded bypowdered carbon wet with sal-ammoniac solu-tion. Sawdust would do in place of the carbonpowder, but would not be quite so good. Thepitch is poured into the top of the battery to pre-vent the evaporation of the moisture. These bat-teries come in a paper box, so they can be placedtogether, but do not let the metal parts come incontact, which would soon Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/st-nicholas-serial-shell-is-the-zinc-plate-to-which-issoldered-a-binding-post-to-hold-the-wire-nextinside-of-this-is-a-porous-paper-lining-for-insula-tion-extending-down-into-this-is-a-solid-carboncylinderany-shape-will-do-surrounded-bypowdered-carbon-wet-with-sal-ammoniac-solu-tion-sawdust-would-do-in-place-of-the-carbonpowder-but-would-not-be-quite-so-good-thepitch-is-poured-into-the-top-of-the-battery-to-pre-vent-the-evaporation-of-the-moisture-these-bat-teries-come-in-a-paper-box-so-they-can-be-placedtogether-but-do-not-let-the-metal-parts-come-incontact-which-would-soon-image338249861.htmlRM2AJ8H45–St Nicholas [serial] . shell is the zinc plate, to which issoldered a binding-post to hold the wire. Next,inside of this, is a porous paper lining for insula-tion. Extending down into this is a solid carboncylinder—any shape will do —surrounded bypowdered carbon wet with sal-ammoniac solu-tion. Sawdust would do in place of the carbonpowder, but would not be quite so good. Thepitch is poured into the top of the battery to pre-vent the evaporation of the moisture. These bat-teries come in a paper box, so they can be placedtogether, but do not let the metal parts come incontact, which would soon
 A popular chemistry; . orns of the hart. It received the nameammonia, by which it is now more generally known,from the temple of Jupiter Ammon, near which sal-ammoniac, one of its compounds, was once manu-factured. The aqua am-monia of the shops,which is merely a strongsolution of the gas inH20, is obtained fromthe incidental productsof the gas-works in largequantities. (See p. 72.)Its pungent odor canoften be detected neardecaying vegetable andanimal matter. Preparation. — NH3 isordinarily prepared byheating sal-ammoniacwith lime.* The reac-tion may be represented as follows : 2NH4C1 + CaO = Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/a-popular-chemistry-orns-of-the-hart-it-received-the-nameammonia-by-which-it-is-now-more-generally-knownfrom-the-temple-of-jupiter-ammon-near-which-sal-ammoniac-one-of-its-compounds-was-once-manu-factured-the-aqua-am-monia-of-the-shopswhich-is-merely-a-strongsolution-of-the-gas-inh20-is-obtained-fromthe-incidental-productsof-the-gas-works-in-largequantities-see-p-72its-pungent-odor-canoften-be-detected-neardecaying-vegetable-andanimal-matter-preparation-nh3-isordinarily-prepared-byheating-sal-ammoniacwith-lime-the-reac-tion-may-be-represented-as-follows-2nh4c1-cao-=-image342707700.html
A popular chemistry; . orns of the hart. It received the nameammonia, by which it is now more generally known,from the temple of Jupiter Ammon, near which sal-ammoniac, one of its compounds, was once manu-factured. The aqua am-monia of the shops,which is merely a strongsolution of the gas inH20, is obtained fromthe incidental productsof the gas-works in largequantities. (See p. 72.)Its pungent odor canoften be detected neardecaying vegetable andanimal matter. Preparation. — NH3 isordinarily prepared byheating sal-ammoniacwith lime.* The reac-tion may be represented as follows : 2NH4C1 + CaO = Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/a-popular-chemistry-orns-of-the-hart-it-received-the-nameammonia-by-which-it-is-now-more-generally-knownfrom-the-temple-of-jupiter-ammon-near-which-sal-ammoniac-one-of-its-compounds-was-once-manu-factured-the-aqua-am-monia-of-the-shopswhich-is-merely-a-strongsolution-of-the-gas-inh20-is-obtained-fromthe-incidental-productsof-the-gas-works-in-largequantities-see-p-72its-pungent-odor-canoften-be-detected-neardecaying-vegetable-andanimal-matter-preparation-nh3-isordinarily-prepared-byheating-sal-ammoniacwith-lime-the-reac-tion-may-be-represented-as-follows-2nh4c1-cao-=-image342707700.htmlRM2AWFK4M–A popular chemistry; . orns of the hart. It received the nameammonia, by which it is now more generally known,from the temple of Jupiter Ammon, near which sal-ammoniac, one of its compounds, was once manu-factured. The aqua am-monia of the shops,which is merely a strongsolution of the gas inH20, is obtained fromthe incidental productsof the gas-works in largequantities. (See p. 72.)Its pungent odor canoften be detected neardecaying vegetable andanimal matter. Preparation. — NH3 isordinarily prepared byheating sal-ammoniacwith lime.* The reac-tion may be represented as follows : 2NH4C1 + CaO =
 Lessons in practical electricity; principles, experiments, and arithmetical problems, an elementary text-book . Fig. 89.—Elements of Carbon Cylinder Cell with Depolarizer.Zinc, manganese dioxide in a porous cup and sal-ammoniac solution. manganese and then sealed in. The zinc rod is prevented fromtouching the carbon by being first inserted through a porce-lain insulator. About 4 to 6 ounces of sal-ammoniac are gen-erally used for cells of ordinary size. The salt is placed inthe jar, water poured in until it is abouttwo-thirds full, and then stirred till all thesalt is dissolved. When the carbo Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/lessons-in-practical-electricity-principles-experiments-and-arithmetical-problems-an-elementary-text-book-fig-89elements-of-carbon-cylinder-cell-with-depolarizerzinc-manganese-dioxide-in-a-porous-cup-and-sal-ammoniac-solution-manganese-and-then-sealed-in-the-zinc-rod-is-prevented-fromtouching-the-carbon-by-being-first-inserted-through-a-porce-lain-insulator-about-4-to-6-ounces-of-sal-ammoniac-are-gen-erally-used-for-cells-of-ordinary-size-the-salt-is-placed-inthe-jar-water-poured-in-until-it-is-abouttwo-thirds-full-and-then-stirred-till-all-thesalt-is-dissolved-when-the-carbo-image340071277.html
Lessons in practical electricity; principles, experiments, and arithmetical problems, an elementary text-book . Fig. 89.—Elements of Carbon Cylinder Cell with Depolarizer.Zinc, manganese dioxide in a porous cup and sal-ammoniac solution. manganese and then sealed in. The zinc rod is prevented fromtouching the carbon by being first inserted through a porce-lain insulator. About 4 to 6 ounces of sal-ammoniac are gen-erally used for cells of ordinary size. The salt is placed inthe jar, water poured in until it is abouttwo-thirds full, and then stirred till all thesalt is dissolved. When the carbo Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/lessons-in-practical-electricity-principles-experiments-and-arithmetical-problems-an-elementary-text-book-fig-89elements-of-carbon-cylinder-cell-with-depolarizerzinc-manganese-dioxide-in-a-porous-cup-and-sal-ammoniac-solution-manganese-and-then-sealed-in-the-zinc-rod-is-prevented-fromtouching-the-carbon-by-being-first-inserted-through-a-porce-lain-insulator-about-4-to-6-ounces-of-sal-ammoniac-are-gen-erally-used-for-cells-of-ordinary-size-the-salt-is-placed-inthe-jar-water-poured-in-until-it-is-abouttwo-thirds-full-and-then-stirred-till-all-thesalt-is-dissolved-when-the-carbo-image340071277.htmlRM2AN7GAN–Lessons in practical electricity; principles, experiments, and arithmetical problems, an elementary text-book . Fig. 89.—Elements of Carbon Cylinder Cell with Depolarizer.Zinc, manganese dioxide in a porous cup and sal-ammoniac solution. manganese and then sealed in. The zinc rod is prevented fromtouching the carbon by being first inserted through a porce-lain insulator. About 4 to 6 ounces of sal-ammoniac are gen-erally used for cells of ordinary size. The salt is placed inthe jar, water poured in until it is abouttwo-thirds full, and then stirred till all thesalt is dissolved. When the carbo
 A text-book on chemistryFor the use of schools and colleges . erent specific gravi-ties, its composition is nearly the same everywhere. This is o^jng partly to the winds and partly to the principle known as the dif-fusion of gases, which may be illustrated asfollows : If two vials, h c, Fig. 235, are rinsedout, h with ammonia and c with hydrochloricacid, each becomes filled with the vapor ofthe liquid used. On placing them mouth tomouth, as in the figure, dense white fumesof sal ammoniac (NH4C1) appear simultane-ously in both. The same effect will take place even thoughbarriers intervene, as m Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/a-text-book-on-chemistryfor-the-use-of-schools-and-colleges-erent-specific-gravi-ties-its-composition-is-nearly-the-same-everywhere-this-is-ojng-partly-to-the-winds-and-partly-to-the-principle-known-as-the-dif-fusion-of-gases-which-may-be-illustrated-asfollows-if-two-vials-h-c-fig-235-are-rinsedout-h-with-ammonia-and-c-with-hydrochloricacid-each-becomes-filled-with-the-vapor-ofthe-liquid-used-on-placing-them-mouth-tomouth-as-in-the-figure-dense-white-fumesof-sal-ammoniac-nh4c1-appear-simultane-ously-in-both-the-same-effect-will-take-place-even-thoughbarriers-intervene-as-m-image343318207.html
A text-book on chemistryFor the use of schools and colleges . erent specific gravi-ties, its composition is nearly the same everywhere. This is o^jng partly to the winds and partly to the principle known as the dif-fusion of gases, which may be illustrated asfollows : If two vials, h c, Fig. 235, are rinsedout, h with ammonia and c with hydrochloricacid, each becomes filled with the vapor ofthe liquid used. On placing them mouth tomouth, as in the figure, dense white fumesof sal ammoniac (NH4C1) appear simultane-ously in both. The same effect will take place even thoughbarriers intervene, as m Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/a-text-book-on-chemistryfor-the-use-of-schools-and-colleges-erent-specific-gravi-ties-its-composition-is-nearly-the-same-everywhere-this-is-ojng-partly-to-the-winds-and-partly-to-the-principle-known-as-the-dif-fusion-of-gases-which-may-be-illustrated-asfollows-if-two-vials-h-c-fig-235-are-rinsedout-h-with-ammonia-and-c-with-hydrochloricacid-each-becomes-filled-with-the-vapor-ofthe-liquid-used-on-placing-them-mouth-tomouth-as-in-the-figure-dense-white-fumesof-sal-ammoniac-nh4c1-appear-simultane-ously-in-both-the-same-effect-will-take-place-even-thoughbarriers-intervene-as-m-image343318207.htmlRM2AXFDTF–A text-book on chemistryFor the use of schools and colleges . erent specific gravi-ties, its composition is nearly the same everywhere. This is o^jng partly to the winds and partly to the principle known as the dif-fusion of gases, which may be illustrated asfollows : If two vials, h c, Fig. 235, are rinsedout, h with ammonia and c with hydrochloricacid, each becomes filled with the vapor ofthe liquid used. On placing them mouth tomouth, as in the figure, dense white fumesof sal ammoniac (NH4C1) appear simultane-ously in both. The same effect will take place even thoughbarriers intervene, as m
![Lectures on nervous diseases from the standpoint of cerebral and spinal localization, and the later methods employed in the diagnosis and treatment of these affections . rs.—The best of this group is the cell devisedby Leclanche. Leclanches Cell (1868).—The carbon ele-ment is ])acked in a porous cup, with the needleform of the black oxide of manganese surround-ing it. This cup is then placed in a glass vessel,containing a rod of zinc and a solution of sal. ammoniac. The cup iscarefully sealed to prevent evaporation and escape of its contents.E. M. F. = 1.48 volt, when the battery is not polari Stock Photo Lectures on nervous diseases from the standpoint of cerebral and spinal localization, and the later methods employed in the diagnosis and treatment of these affections . rs.—The best of this group is the cell devisedby Leclanche. Leclanches Cell (1868).—The carbon ele-ment is ])acked in a porous cup, with the needleform of the black oxide of manganese surround-ing it. This cup is then placed in a glass vessel,containing a rod of zinc and a solution of sal. ammoniac. The cup iscarefully sealed to prevent evaporation and escape of its contents.E. M. F. = 1.48 volt, when the battery is not polari Stock Photo](https://c8.alamy.com/comp/2AWH2XY/lectures-on-nervous-diseases-from-the-standpoint-of-cerebral-and-spinal-localization-and-the-later-methods-employed-in-the-diagnosis-and-treatment-of-these-affections-rsthe-best-of-this-group-is-the-cell-devisedby-leclanche-leclanches-cell-1868the-carbon-ele-ment-is-acked-in-a-porous-cup-with-the-needleform-of-the-black-oxide-of-manganese-surround-ing-it-this-cup-is-then-placed-in-a-glass-vesselcontaining-a-rod-of-zinc-and-a-solution-of-sal-ammoniac-the-cup-iscarefully-sealed-to-prevent-evaporation-and-escape-of-its-contentse-m-f-=-148-volt-when-the-battery-is-not-polari-2AWH2XY.jpg) Lectures on nervous diseases from the standpoint of cerebral and spinal localization, and the later methods employed in the diagnosis and treatment of these affections . rs.—The best of this group is the cell devisedby Leclanche. Leclanches Cell (1868).—The carbon ele-ment is ])acked in a porous cup, with the needleform of the black oxide of manganese surround-ing it. This cup is then placed in a glass vessel,containing a rod of zinc and a solution of sal. ammoniac. The cup iscarefully sealed to prevent evaporation and escape of its contents.E. M. F. = 1.48 volt, when the battery is not polari Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/lectures-on-nervous-diseases-from-the-standpoint-of-cerebral-and-spinal-localization-and-the-later-methods-employed-in-the-diagnosis-and-treatment-of-these-affections-rsthe-best-of-this-group-is-the-cell-devisedby-leclanche-leclanches-cell-1868the-carbon-ele-ment-is-acked-in-a-porous-cup-with-the-needleform-of-the-black-oxide-of-manganese-surround-ing-it-this-cup-is-then-placed-in-a-glass-vesselcontaining-a-rod-of-zinc-and-a-solution-of-sal-ammoniac-the-cup-iscarefully-sealed-to-prevent-evaporation-and-escape-of-its-contentse-m-f-=-148-volt-when-the-battery-is-not-polari-image342738899.html
Lectures on nervous diseases from the standpoint of cerebral and spinal localization, and the later methods employed in the diagnosis and treatment of these affections . rs.—The best of this group is the cell devisedby Leclanche. Leclanches Cell (1868).—The carbon ele-ment is ])acked in a porous cup, with the needleform of the black oxide of manganese surround-ing it. This cup is then placed in a glass vessel,containing a rod of zinc and a solution of sal. ammoniac. The cup iscarefully sealed to prevent evaporation and escape of its contents.E. M. F. = 1.48 volt, when the battery is not polari Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/lectures-on-nervous-diseases-from-the-standpoint-of-cerebral-and-spinal-localization-and-the-later-methods-employed-in-the-diagnosis-and-treatment-of-these-affections-rsthe-best-of-this-group-is-the-cell-devisedby-leclanche-leclanches-cell-1868the-carbon-ele-ment-is-acked-in-a-porous-cup-with-the-needleform-of-the-black-oxide-of-manganese-surround-ing-it-this-cup-is-then-placed-in-a-glass-vesselcontaining-a-rod-of-zinc-and-a-solution-of-sal-ammoniac-the-cup-iscarefully-sealed-to-prevent-evaporation-and-escape-of-its-contentse-m-f-=-148-volt-when-the-battery-is-not-polari-image342738899.htmlRM2AWH2XY–Lectures on nervous diseases from the standpoint of cerebral and spinal localization, and the later methods employed in the diagnosis and treatment of these affections . rs.—The best of this group is the cell devisedby Leclanche. Leclanches Cell (1868).—The carbon ele-ment is ])acked in a porous cup, with the needleform of the black oxide of manganese surround-ing it. This cup is then placed in a glass vessel,containing a rod of zinc and a solution of sal. ammoniac. The cup iscarefully sealed to prevent evaporation and escape of its contents.E. M. F. = 1.48 volt, when the battery is not polari
 A text-book of the diseases of the ear and adjacent organs . ch is attached to a double balloon, are connected by means of short piecesof india-rubber tubing with two bent glass tubes, r, ?-, each of which carriesa perforated rubber stopper at its other extremity, these stoppers beingaccurately fitted into the glass tubes a, n. On pressing the double balloon, 112 INJECTION OF VAPOURS INTO THE MIDDLE EAR. vapours of hydrochloric acid and ammonia are forced into the lower sectionof the Y-shaped tube, where they unite to form sal-ammoniac vapour, whichbeing purified in its passage through the wat Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/a-text-book-of-the-diseases-of-the-ear-and-adjacent-organs-ch-is-attached-to-a-double-balloon-are-connected-by-means-of-short-piecesof-india-rubber-tubing-with-two-bent-glass-tubes-r-each-of-which-carriesa-perforated-rubber-stopper-at-its-other-extremity-these-stoppers-beingaccurately-fitted-into-the-glass-tubes-a-n-on-pressing-the-double-balloon-112-injection-of-vapours-into-the-middle-ear-vapours-of-hydrochloric-acid-and-ammonia-are-forced-into-the-lower-sectionof-the-y-shaped-tube-where-they-unite-to-form-sal-ammoniac-vapour-whichbeing-purified-in-its-passage-through-the-wat-image340026706.html
A text-book of the diseases of the ear and adjacent organs . ch is attached to a double balloon, are connected by means of short piecesof india-rubber tubing with two bent glass tubes, r, ?-, each of which carriesa perforated rubber stopper at its other extremity, these stoppers beingaccurately fitted into the glass tubes a, n. On pressing the double balloon, 112 INJECTION OF VAPOURS INTO THE MIDDLE EAR. vapours of hydrochloric acid and ammonia are forced into the lower sectionof the Y-shaped tube, where they unite to form sal-ammoniac vapour, whichbeing purified in its passage through the wat Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/a-text-book-of-the-diseases-of-the-ear-and-adjacent-organs-ch-is-attached-to-a-double-balloon-are-connected-by-means-of-short-piecesof-india-rubber-tubing-with-two-bent-glass-tubes-r-each-of-which-carriesa-perforated-rubber-stopper-at-its-other-extremity-these-stoppers-beingaccurately-fitted-into-the-glass-tubes-a-n-on-pressing-the-double-balloon-112-injection-of-vapours-into-the-middle-ear-vapours-of-hydrochloric-acid-and-ammonia-are-forced-into-the-lower-sectionof-the-y-shaped-tube-where-they-unite-to-form-sal-ammoniac-vapour-whichbeing-purified-in-its-passage-through-the-wat-image340026706.htmlRM2AN5FEX–A text-book of the diseases of the ear and adjacent organs . ch is attached to a double balloon, are connected by means of short piecesof india-rubber tubing with two bent glass tubes, r, ?-, each of which carriesa perforated rubber stopper at its other extremity, these stoppers beingaccurately fitted into the glass tubes a, n. On pressing the double balloon, 112 INJECTION OF VAPOURS INTO THE MIDDLE EAR. vapours of hydrochloric acid and ammonia are forced into the lower sectionof the Y-shaped tube, where they unite to form sal-ammoniac vapour, whichbeing purified in its passage through the wat
 Lessons in practical electricity; principles, experiments, and arithmetical problems, an elementary text-book . h holds the other element (carbon)and the exciting mixture. The carbon rodor plate occupies about one-half the spacein the cell, and the space between the carbonand zinc cylinder is tilled with the followingmixture, the proportions being by weight:Oxide of zinc, 1 part ; sal-ammoniac, 1 part;plaster, 3 parts; chloride of zinc, 1 part;water, 2 parts. Dry cells being portable,are very convenient for use where only an intermittentcurrent is required. The E. M. F. is about 1.4 volts. 94. Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/lessons-in-practical-electricity-principles-experiments-and-arithmetical-problems-an-elementary-text-book-h-holds-the-other-element-carbonand-the-exciting-mixture-the-carbon-rodor-plate-occupies-about-one-half-the-spacein-the-cell-and-the-space-between-the-carbonand-zinc-cylinder-is-tilled-with-the-followingmixture-the-proportions-being-by-weightoxide-of-zinc-1-part-sal-ammoniac-1-partplaster-3-parts-chloride-of-zinc-1-partwater-2-parts-dry-cells-being-portableare-very-convenient-for-use-where-only-an-intermittentcurrent-is-required-the-e-m-f-is-about-14-volts-94-image340070615.html
Lessons in practical electricity; principles, experiments, and arithmetical problems, an elementary text-book . h holds the other element (carbon)and the exciting mixture. The carbon rodor plate occupies about one-half the spacein the cell, and the space between the carbonand zinc cylinder is tilled with the followingmixture, the proportions being by weight:Oxide of zinc, 1 part ; sal-ammoniac, 1 part;plaster, 3 parts; chloride of zinc, 1 part;water, 2 parts. Dry cells being portable,are very convenient for use where only an intermittentcurrent is required. The E. M. F. is about 1.4 volts. 94. Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/lessons-in-practical-electricity-principles-experiments-and-arithmetical-problems-an-elementary-text-book-h-holds-the-other-element-carbonand-the-exciting-mixture-the-carbon-rodor-plate-occupies-about-one-half-the-spacein-the-cell-and-the-space-between-the-carbonand-zinc-cylinder-is-tilled-with-the-followingmixture-the-proportions-being-by-weightoxide-of-zinc-1-part-sal-ammoniac-1-partplaster-3-parts-chloride-of-zinc-1-partwater-2-parts-dry-cells-being-portableare-very-convenient-for-use-where-only-an-intermittentcurrent-is-required-the-e-m-f-is-about-14-volts-94-image340070615.htmlRM2AN7FF3–Lessons in practical electricity; principles, experiments, and arithmetical problems, an elementary text-book . h holds the other element (carbon)and the exciting mixture. The carbon rodor plate occupies about one-half the spacein the cell, and the space between the carbonand zinc cylinder is tilled with the followingmixture, the proportions being by weight:Oxide of zinc, 1 part ; sal-ammoniac, 1 part;plaster, 3 parts; chloride of zinc, 1 part;water, 2 parts. Dry cells being portable,are very convenient for use where only an intermittentcurrent is required. The E. M. F. is about 1.4 volts. 94.
 A supplement to Ures Dictionary of Arts, Manufactures, and Mines, : containing a clear exposition of their principles and practice. . rine worts during fermentation, by the conversion of the sugar into alcohol and car-bonic acid. AURUM MUSIVUM or JIOSAICUif. Mosaic Gold.—For the preparation of Mo.saiegold, the following process is recommended by Woulfe. An amalgam of 2 parts of tin and1 part of mercury is prepared in a liot crucil)le, and triturated with 1 part of sal-ammoniac,and 1 part of flower of sulphur; the mixture is sulilimed in a glass flask ufion thesand l)ath. In breaking the flask Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/a-supplement-to-ures-dictionary-of-arts-manufactures-and-mines-containing-a-clear-exposition-of-their-principles-and-practice-rine-worts-during-fermentation-by-the-conversion-of-the-sugar-into-alcohol-and-car-bonic-acid-aurum-musivum-or-jiosaicuif-mosaic-goldfor-the-preparation-of-mosaiegold-the-following-process-is-recommended-by-woulfe-an-amalgam-of-2-parts-of-tin-and1-part-of-mercury-is-prepared-in-a-liot-crucille-and-triturated-with-1-part-of-sal-ammoniacand-1-part-of-flower-of-sulphur-the-mixture-is-sulilimed-in-a-glass-flask-ufion-thesand-lath-in-breaking-the-flask-image340013816.html
A supplement to Ures Dictionary of Arts, Manufactures, and Mines, : containing a clear exposition of their principles and practice. . rine worts during fermentation, by the conversion of the sugar into alcohol and car-bonic acid. AURUM MUSIVUM or JIOSAICUif. Mosaic Gold.—For the preparation of Mo.saiegold, the following process is recommended by Woulfe. An amalgam of 2 parts of tin and1 part of mercury is prepared in a liot crucil)le, and triturated with 1 part of sal-ammoniac,and 1 part of flower of sulphur; the mixture is sulilimed in a glass flask ufion thesand l)ath. In breaking the flask Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/a-supplement-to-ures-dictionary-of-arts-manufactures-and-mines-containing-a-clear-exposition-of-their-principles-and-practice-rine-worts-during-fermentation-by-the-conversion-of-the-sugar-into-alcohol-and-car-bonic-acid-aurum-musivum-or-jiosaicuif-mosaic-goldfor-the-preparation-of-mosaiegold-the-following-process-is-recommended-by-woulfe-an-amalgam-of-2-parts-of-tin-and1-part-of-mercury-is-prepared-in-a-liot-crucille-and-triturated-with-1-part-of-sal-ammoniacand-1-part-of-flower-of-sulphur-the-mixture-is-sulilimed-in-a-glass-flask-ufion-thesand-lath-in-breaking-the-flask-image340013816.htmlRM2AN4Y2G–A supplement to Ures Dictionary of Arts, Manufactures, and Mines, : containing a clear exposition of their principles and practice. . rine worts during fermentation, by the conversion of the sugar into alcohol and car-bonic acid. AURUM MUSIVUM or JIOSAICUif. Mosaic Gold.—For the preparation of Mo.saiegold, the following process is recommended by Woulfe. An amalgam of 2 parts of tin and1 part of mercury is prepared in a liot crucil)le, and triturated with 1 part of sal-ammoniac,and 1 part of flower of sulphur; the mixture is sulilimed in a glass flask ufion thesand l)ath. In breaking the flask
 Wells's principles and applications of chemistry; . cal importance. 369. Chloride of Nitroge n.—The single compound which chlorin©is known to form with nitrogen, is especially worthy of not© as probably themost dangerous of all chemical combinations. When a bottle of chlorine, perfectly free from greasy matter, is invertedover a leaden dish containing a solution of 1 part of sal-ammoniac (NH4Ci) in12 parts of water—the mouth of the bottle slightly dipping beneath the sur-face—-drops of an oily-looking substance will gradually form upon the liquidand fall to the bottom of the dish,—chlorine slo Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/wellss-principles-and-applications-of-chemistry-cal-importance-369-chloride-of-nitroge-nthe-single-compound-which-chlorinis-known-to-form-with-nitrogen-is-especially-worthy-of-not-as-probably-themost-dangerous-of-all-chemical-combinations-when-a-bottle-of-chlorine-perfectly-free-from-greasy-matter-is-invertedover-a-leaden-dish-containing-a-solution-of-1-part-of-sal-ammoniac-nh4ci-in12-parts-of-waterthe-mouth-of-the-bottle-slightly-dipping-beneath-the-sur-face-drops-of-an-oily-looking-substance-will-gradually-form-upon-the-liquidand-fall-to-the-bottom-of-the-dishchlorine-slo-image342839410.html
Wells's principles and applications of chemistry; . cal importance. 369. Chloride of Nitroge n.—The single compound which chlorin©is known to form with nitrogen, is especially worthy of not© as probably themost dangerous of all chemical combinations. When a bottle of chlorine, perfectly free from greasy matter, is invertedover a leaden dish containing a solution of 1 part of sal-ammoniac (NH4Ci) in12 parts of water—the mouth of the bottle slightly dipping beneath the sur-face—-drops of an oily-looking substance will gradually form upon the liquidand fall to the bottom of the dish,—chlorine slo Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/wellss-principles-and-applications-of-chemistry-cal-importance-369-chloride-of-nitroge-nthe-single-compound-which-chlorinis-known-to-form-with-nitrogen-is-especially-worthy-of-not-as-probably-themost-dangerous-of-all-chemical-combinations-when-a-bottle-of-chlorine-perfectly-free-from-greasy-matter-is-invertedover-a-leaden-dish-containing-a-solution-of-1-part-of-sal-ammoniac-nh4ci-in12-parts-of-waterthe-mouth-of-the-bottle-slightly-dipping-beneath-the-sur-face-drops-of-an-oily-looking-substance-will-gradually-form-upon-the-liquidand-fall-to-the-bottom-of-the-dishchlorine-slo-image342839410.htmlRM2AWNK4J–Wells's principles and applications of chemistry; . cal importance. 369. Chloride of Nitroge n.—The single compound which chlorin©is known to form with nitrogen, is especially worthy of not© as probably themost dangerous of all chemical combinations. When a bottle of chlorine, perfectly free from greasy matter, is invertedover a leaden dish containing a solution of 1 part of sal-ammoniac (NH4Ci) in12 parts of water—the mouth of the bottle slightly dipping beneath the sur-face—-drops of an oily-looking substance will gradually form upon the liquidand fall to the bottom of the dish,—chlorine slo
 Lessons in practical electricity; principles, experiments, and arithmetical problems, an elementary text-book . Fig. 89.—Elements of Carbon Cylinder Cell with Depolarizer.Zinc, manganese dioxide in a porous cup and sal-ammoniac solution. manganese and then sealed in. The zinc rod is prevented fromtouching the carbon by being first inserted through a porce-lain insulator. About 4 to 6 ounces of sal-ammoniac are gen-erally used for cells of ordinary size. The salt is placed inthe jar, water poured in until it is abouttwo-thirds full, and then stirred till all thesalt is dissolved. When the carbo Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/lessons-in-practical-electricity-principles-experiments-and-arithmetical-problems-an-elementary-text-book-fig-89elements-of-carbon-cylinder-cell-with-depolarizerzinc-manganese-dioxide-in-a-porous-cup-and-sal-ammoniac-solution-manganese-and-then-sealed-in-the-zinc-rod-is-prevented-fromtouching-the-carbon-by-being-first-inserted-through-a-porce-lain-insulator-about-4-to-6-ounces-of-sal-ammoniac-are-gen-erally-used-for-cells-of-ordinary-size-the-salt-is-placed-inthe-jar-water-poured-in-until-it-is-abouttwo-thirds-full-and-then-stirred-till-all-thesalt-is-dissolved-when-the-carbo-image340071951.html
Lessons in practical electricity; principles, experiments, and arithmetical problems, an elementary text-book . Fig. 89.—Elements of Carbon Cylinder Cell with Depolarizer.Zinc, manganese dioxide in a porous cup and sal-ammoniac solution. manganese and then sealed in. The zinc rod is prevented fromtouching the carbon by being first inserted through a porce-lain insulator. About 4 to 6 ounces of sal-ammoniac are gen-erally used for cells of ordinary size. The salt is placed inthe jar, water poured in until it is abouttwo-thirds full, and then stirred till all thesalt is dissolved. When the carbo Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/lessons-in-practical-electricity-principles-experiments-and-arithmetical-problems-an-elementary-text-book-fig-89elements-of-carbon-cylinder-cell-with-depolarizerzinc-manganese-dioxide-in-a-porous-cup-and-sal-ammoniac-solution-manganese-and-then-sealed-in-the-zinc-rod-is-prevented-fromtouching-the-carbon-by-being-first-inserted-through-a-porce-lain-insulator-about-4-to-6-ounces-of-sal-ammoniac-are-gen-erally-used-for-cells-of-ordinary-size-the-salt-is-placed-inthe-jar-water-poured-in-until-it-is-abouttwo-thirds-full-and-then-stirred-till-all-thesalt-is-dissolved-when-the-carbo-image340071951.htmlRM2AN7H6R–Lessons in practical electricity; principles, experiments, and arithmetical problems, an elementary text-book . Fig. 89.—Elements of Carbon Cylinder Cell with Depolarizer.Zinc, manganese dioxide in a porous cup and sal-ammoniac solution. manganese and then sealed in. The zinc rod is prevented fromtouching the carbon by being first inserted through a porce-lain insulator. About 4 to 6 ounces of sal-ammoniac are gen-erally used for cells of ordinary size. The salt is placed inthe jar, water poured in until it is abouttwo-thirds full, and then stirred till all thesalt is dissolved. When the carbo
 Scientific amusements . Apparatus for obtaining laugiiing-gas. which yield ammonia, which is a colourless gas of strongodour and taste now obtained from gas-works. To obtain AMMONIA heat equal parts of chloride ofammonia (sal ammoniac) and quick-lime powdered (seepage 87). The gas must be collected over mercury, be-cause it is very soluble in water. Ammonia is useful torestore tipsy people and fainting ladies. A solution ofammonia is used for cauteries. Ammoniacal gas is re-markable for its solubility in water. To prepare thesolution the gas is forced thmugh a series of flasks. Thetubes carryi Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/scientific-amusements-apparatus-for-obtaining-laugiiing-gas-which-yield-ammonia-which-is-a-colourless-gas-of-strongodour-and-taste-now-obtained-from-gas-works-to-obtain-ammonia-heat-equal-parts-of-chloride-ofammonia-sal-ammoniac-and-quick-lime-powdered-seepage-87-the-gas-must-be-collected-over-mercury-be-cause-it-is-very-soluble-in-water-ammonia-is-useful-torestore-tipsy-people-and-fainting-ladies-a-solution-ofammonia-is-used-for-cauteries-ammoniacal-gas-is-re-markable-for-its-solubility-in-water-to-prepare-thesolution-the-gas-is-forced-thmugh-a-series-of-flasks-thetubes-carryi-image343313852.html
Scientific amusements . Apparatus for obtaining laugiiing-gas. which yield ammonia, which is a colourless gas of strongodour and taste now obtained from gas-works. To obtain AMMONIA heat equal parts of chloride ofammonia (sal ammoniac) and quick-lime powdered (seepage 87). The gas must be collected over mercury, be-cause it is very soluble in water. Ammonia is useful torestore tipsy people and fainting ladies. A solution ofammonia is used for cauteries. Ammoniacal gas is re-markable for its solubility in water. To prepare thesolution the gas is forced thmugh a series of flasks. Thetubes carryi Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/scientific-amusements-apparatus-for-obtaining-laugiiing-gas-which-yield-ammonia-which-is-a-colourless-gas-of-strongodour-and-taste-now-obtained-from-gas-works-to-obtain-ammonia-heat-equal-parts-of-chloride-ofammonia-sal-ammoniac-and-quick-lime-powdered-seepage-87-the-gas-must-be-collected-over-mercury-be-cause-it-is-very-soluble-in-water-ammonia-is-useful-torestore-tipsy-people-and-fainting-ladies-a-solution-ofammonia-is-used-for-cauteries-ammoniacal-gas-is-re-markable-for-its-solubility-in-water-to-prepare-thesolution-the-gas-is-forced-thmugh-a-series-of-flasks-thetubes-carryi-image343313852.htmlRM2AXF890–Scientific amusements . Apparatus for obtaining laugiiing-gas. which yield ammonia, which is a colourless gas of strongodour and taste now obtained from gas-works. To obtain AMMONIA heat equal parts of chloride ofammonia (sal ammoniac) and quick-lime powdered (seepage 87). The gas must be collected over mercury, be-cause it is very soluble in water. Ammonia is useful torestore tipsy people and fainting ladies. A solution ofammonia is used for cauteries. Ammoniacal gas is re-markable for its solubility in water. To prepare thesolution the gas is forced thmugh a series of flasks. Thetubes carryi
 A manual of practical medical electricity : the Röntgen rays and Finsen light . es are : 1. It requires scarcely any looking after. 2. It will last with fair use for an indefinite period. 3. It does not deteriorate through inactivity. 4. Though it polarizes rapidly on short circuit, yetit will yield during ordinary galvanization a practicallyconstant current for from ten to twenty minutes. 5. It is cleanly, and it yields no fumes. Forms of Primary Cells 37 It is better in charging the cell to dissolve the sal-ammoniac (which should be pure) in warm water in a jug,and to half fill the cell with Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/a-manual-of-practical-medical-electricity-the-rntgen-rays-and-finsen-light-es-are-1-it-requires-scarcely-any-looking-after-2-it-will-last-with-fair-use-for-an-indefinite-period-3-it-does-not-deteriorate-through-inactivity-4-though-it-polarizes-rapidly-on-short-circuit-yetit-will-yield-during-ordinary-galvanization-a-practicallyconstant-current-for-from-ten-to-twenty-minutes-5-it-is-cleanly-and-it-yields-no-fumes-forms-of-primary-cells-37-it-is-better-in-charging-the-cell-to-dissolve-the-sal-ammoniac-which-should-be-pure-in-warm-water-in-a-jugand-to-half-fill-the-cell-with-image338193175.html
A manual of practical medical electricity : the Röntgen rays and Finsen light . es are : 1. It requires scarcely any looking after. 2. It will last with fair use for an indefinite period. 3. It does not deteriorate through inactivity. 4. Though it polarizes rapidly on short circuit, yetit will yield during ordinary galvanization a practicallyconstant current for from ten to twenty minutes. 5. It is cleanly, and it yields no fumes. Forms of Primary Cells 37 It is better in charging the cell to dissolve the sal-ammoniac (which should be pure) in warm water in a jug,and to half fill the cell with Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/a-manual-of-practical-medical-electricity-the-rntgen-rays-and-finsen-light-es-are-1-it-requires-scarcely-any-looking-after-2-it-will-last-with-fair-use-for-an-indefinite-period-3-it-does-not-deteriorate-through-inactivity-4-though-it-polarizes-rapidly-on-short-circuit-yetit-will-yield-during-ordinary-galvanization-a-practicallyconstant-current-for-from-ten-to-twenty-minutes-5-it-is-cleanly-and-it-yields-no-fumes-forms-of-primary-cells-37-it-is-better-in-charging-the-cell-to-dissolve-the-sal-ammoniac-which-should-be-pure-in-warm-water-in-a-jugand-to-half-fill-the-cell-with-image338193175.htmlRM2AJ60RK–A manual of practical medical electricity : the Röntgen rays and Finsen light . es are : 1. It requires scarcely any looking after. 2. It will last with fair use for an indefinite period. 3. It does not deteriorate through inactivity. 4. Though it polarizes rapidly on short circuit, yetit will yield during ordinary galvanization a practicallyconstant current for from ten to twenty minutes. 5. It is cleanly, and it yields no fumes. Forms of Primary Cells 37 It is better in charging the cell to dissolve the sal-ammoniac (which should be pure) in warm water in a jug,and to half fill the cell with
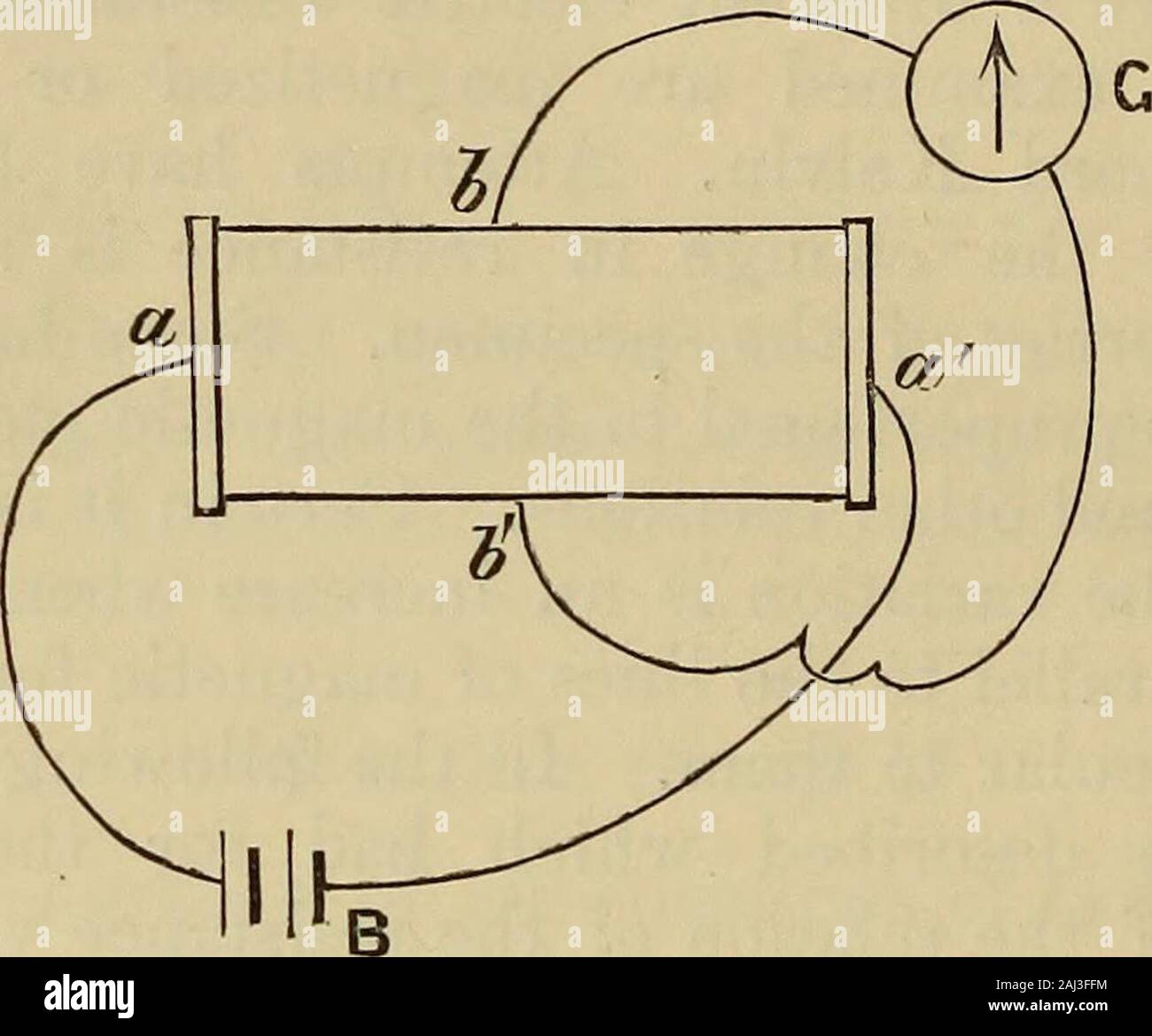 The London, Edinburgh and Dublin philosophical magazine and journal of science . from a solution of fourparts of iron vitriol and three parts sal ammoniac in thirtyparts water. The specimens used were cut in the form of a rectangleusually 9 mm. long by 7 or 8 mm. broad; the thickness ofthe films varied from ^-^-0 of a mm. to -r^ of a mm. Carewas taken to have the thickness of each film as uniform aspossible. To measure the Hall effect the film had the usual twoprimary electrodes soldered along the whole extent of theends, wires from a secondary battery of two cells were soldered * Communicated Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/the-london-edinburgh-and-dublin-philosophical-magazine-and-journal-of-science-from-a-solution-of-fourparts-of-iron-vitriol-and-three-parts-sal-ammoniac-in-thirtyparts-water-the-specimens-used-were-cut-in-the-form-of-a-rectangleusually-9-mm-long-by-7-or-8-mm-broad-the-thickness-ofthe-films-varied-from-0-of-a-mm-to-r-of-a-mm-carewas-taken-to-have-the-thickness-of-each-film-as-uniform-aspossible-to-measure-the-hall-effect-the-film-had-the-usual-twoprimary-electrodes-soldered-along-the-whole-extent-of-theends-wires-from-a-secondary-battery-of-two-cells-were-soldered-communicated-image338138856.html
The London, Edinburgh and Dublin philosophical magazine and journal of science . from a solution of fourparts of iron vitriol and three parts sal ammoniac in thirtyparts water. The specimens used were cut in the form of a rectangleusually 9 mm. long by 7 or 8 mm. broad; the thickness ofthe films varied from ^-^-0 of a mm. to -r^ of a mm. Carewas taken to have the thickness of each film as uniform aspossible. To measure the Hall effect the film had the usual twoprimary electrodes soldered along the whole extent of theends, wires from a secondary battery of two cells were soldered * Communicated Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/the-london-edinburgh-and-dublin-philosophical-magazine-and-journal-of-science-from-a-solution-of-fourparts-of-iron-vitriol-and-three-parts-sal-ammoniac-in-thirtyparts-water-the-specimens-used-were-cut-in-the-form-of-a-rectangleusually-9-mm-long-by-7-or-8-mm-broad-the-thickness-ofthe-films-varied-from-0-of-a-mm-to-r-of-a-mm-carewas-taken-to-have-the-thickness-of-each-film-as-uniform-aspossible-to-measure-the-hall-effect-the-film-had-the-usual-twoprimary-electrodes-soldered-along-the-whole-extent-of-theends-wires-from-a-secondary-battery-of-two-cells-were-soldered-communicated-image338138856.htmlRM2AJ3FFM–The London, Edinburgh and Dublin philosophical magazine and journal of science . from a solution of fourparts of iron vitriol and three parts sal ammoniac in thirtyparts water. The specimens used were cut in the form of a rectangleusually 9 mm. long by 7 or 8 mm. broad; the thickness ofthe films varied from ^-^-0 of a mm. to -r^ of a mm. Carewas taken to have the thickness of each film as uniform aspossible. To measure the Hall effect the film had the usual twoprimary electrodes soldered along the whole extent of theends, wires from a secondary battery of two cells were soldered * Communicated
 Chemistry of the household . Fig. 29. A Battery of Cells Connected iu Series. The zinc is gradually changed to zinc chloride, atthe expense of the ammonium chloride, and after atime bpth the zinc and the ammonium chloride mustbe renewed. In renewing the battery, the jars should becleaned out carefully and the zincs renewed if theyare completely eaten through. A quarter of a poundof pure ammonium chloride (sal-ammoniac) is dis-solved in enough water to about half fill a jar. Whenthe carbon and the zinc are replaced, this will bringthe liquid up to two inches from the top. The jarshould not be f Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/chemistry-of-the-household-fig-29-a-battery-of-cells-connected-iu-series-the-zinc-is-gradually-changed-to-zinc-chloride-atthe-expense-of-the-ammonium-chloride-and-after-atime-bpth-the-zinc-and-the-ammonium-chloride-mustbe-renewed-in-renewing-the-battery-the-jars-should-becleaned-out-carefully-and-the-zincs-renewed-if-theyare-completely-eaten-through-a-quarter-of-a-poundof-pure-ammonium-chloride-sal-ammoniac-is-dis-solved-in-enough-water-to-about-half-fill-a-jar-whenthe-carbon-and-the-zinc-are-replaced-this-will-bringthe-liquid-up-to-two-inches-from-the-top-the-jarshould-not-be-f-image343299765.html
Chemistry of the household . Fig. 29. A Battery of Cells Connected iu Series. The zinc is gradually changed to zinc chloride, atthe expense of the ammonium chloride, and after atime bpth the zinc and the ammonium chloride mustbe renewed. In renewing the battery, the jars should becleaned out carefully and the zincs renewed if theyare completely eaten through. A quarter of a poundof pure ammonium chloride (sal-ammoniac) is dis-solved in enough water to about half fill a jar. Whenthe carbon and the zinc are replaced, this will bringthe liquid up to two inches from the top. The jarshould not be f Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/chemistry-of-the-household-fig-29-a-battery-of-cells-connected-iu-series-the-zinc-is-gradually-changed-to-zinc-chloride-atthe-expense-of-the-ammonium-chloride-and-after-atime-bpth-the-zinc-and-the-ammonium-chloride-mustbe-renewed-in-renewing-the-battery-the-jars-should-becleaned-out-carefully-and-the-zincs-renewed-if-theyare-completely-eaten-through-a-quarter-of-a-poundof-pure-ammonium-chloride-sal-ammoniac-is-dis-solved-in-enough-water-to-about-half-fill-a-jar-whenthe-carbon-and-the-zinc-are-replaced-this-will-bringthe-liquid-up-to-two-inches-from-the-top-the-jarshould-not-be-f-image343299765.htmlRM2AXEJ9W–Chemistry of the household . Fig. 29. A Battery of Cells Connected iu Series. The zinc is gradually changed to zinc chloride, atthe expense of the ammonium chloride, and after atime bpth the zinc and the ammonium chloride mustbe renewed. In renewing the battery, the jars should becleaned out carefully and the zincs renewed if theyare completely eaten through. A quarter of a poundof pure ammonium chloride (sal-ammoniac) is dis-solved in enough water to about half fill a jar. Whenthe carbon and the zinc are replaced, this will bringthe liquid up to two inches from the top. The jarshould not be f
 A practical treatise on the manufacture of colors for painting : comprising the origin, definition, and classification of colors; the treatment of the raw materials .. etc. . llows of a thorough imita-tion of medals, statuettes, vases, etc. The plaster,which has been thus prepared, resists dampness per-fectly well, and becomes v^n-y durable. SECTION YIII. GREEN BRONZE. Take- Strong vinegar 1 litre. Mineral green 15 grammes. Umber . . i . 15 Sal ammoniac 15 Gum Arabic 15 Avignon berries 60 Green copperas 15 And about 85 grammes of green oats, if they can behad, but they are not absolutely neces Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/a-practical-treatise-on-the-manufacture-of-colors-for-painting-comprising-the-origin-definition-and-classification-of-colors-the-treatment-of-the-raw-materials-etc-llows-of-a-thorough-imita-tion-of-medals-statuettes-vases-etc-the-plasterwhich-has-been-thus-prepared-resists-dampness-per-fectly-well-and-becomes-vn-y-durable-section-yiii-green-bronze-take-strong-vinegar-1-litre-mineral-green-15-grammes-umber-i-15-sal-ammoniac-15-gum-arabic-15-avignon-berries-60-green-copperas-15-and-about-85-grammes-of-green-oats-if-they-can-behad-but-they-are-not-absolutely-neces-image338386013.html
A practical treatise on the manufacture of colors for painting : comprising the origin, definition, and classification of colors; the treatment of the raw materials .. etc. . llows of a thorough imita-tion of medals, statuettes, vases, etc. The plaster,which has been thus prepared, resists dampness per-fectly well, and becomes v^n-y durable. SECTION YIII. GREEN BRONZE. Take- Strong vinegar 1 litre. Mineral green 15 grammes. Umber . . i . 15 Sal ammoniac 15 Gum Arabic 15 Avignon berries 60 Green copperas 15 And about 85 grammes of green oats, if they can behad, but they are not absolutely neces Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/a-practical-treatise-on-the-manufacture-of-colors-for-painting-comprising-the-origin-definition-and-classification-of-colors-the-treatment-of-the-raw-materials-etc-llows-of-a-thorough-imita-tion-of-medals-statuettes-vases-etc-the-plasterwhich-has-been-thus-prepared-resists-dampness-per-fectly-well-and-becomes-vn-y-durable-section-yiii-green-bronze-take-strong-vinegar-1-litre-mineral-green-15-grammes-umber-i-15-sal-ammoniac-15-gum-arabic-15-avignon-berries-60-green-copperas-15-and-about-85-grammes-of-green-oats-if-they-can-behad-but-they-are-not-absolutely-neces-image338386013.htmlRM2AJEPPN–A practical treatise on the manufacture of colors for painting : comprising the origin, definition, and classification of colors; the treatment of the raw materials .. etc. . llows of a thorough imita-tion of medals, statuettes, vases, etc. The plaster,which has been thus prepared, resists dampness per-fectly well, and becomes v^n-y durable. SECTION YIII. GREEN BRONZE. Take- Strong vinegar 1 litre. Mineral green 15 grammes. Umber . . i . 15 Sal ammoniac 15 Gum Arabic 15 Avignon berries 60 Green copperas 15 And about 85 grammes of green oats, if they can behad, but they are not absolutely neces
 The Physician and pharmaceutist . solution : Camphor 3 ij jSTitre 3 iss Sal Ammoniac 3 j Proof Spirit I ij3^. Solve. The tube maj^ be tied ovexwith bladder ifreciuired. As a sign of fine weather, the sediment of whiteflakes will settle near to the bottom of the tube, whilethe liquid will be quite transparent above. As a signof rain, the matter will rise to the surface of the so-lution. At the approach of a dorm, the matter wiUfloat on the surface of the solution in the form ofwhite flakes, and the fluid will appear in a state offermentation. During froit, the solution will pre-sent a starry ap Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/the-physician-and-pharmaceutist-solution-camphor-3-ij-jstitre-3-iss-sal-ammoniac-3-j-proof-spirit-i-ij3-solve-the-tube-maj-be-tied-ovexwith-bladder-ifreciuired-as-a-sign-of-fine-weather-the-sediment-of-whiteflakes-will-settle-near-to-the-bottom-of-the-tube-whilethe-liquid-will-be-quite-transparent-above-as-a-signof-rain-the-matter-will-rise-to-the-surface-of-the-so-lution-at-the-approach-of-a-dorm-the-matter-wiufloat-on-the-surface-of-the-solution-in-the-form-ofwhite-flakes-and-the-fluid-will-appear-in-a-state-offermentation-during-froit-the-solution-will-pre-sent-a-starry-ap-image338222956.html
The Physician and pharmaceutist . solution : Camphor 3 ij jSTitre 3 iss Sal Ammoniac 3 j Proof Spirit I ij3^. Solve. The tube maj^ be tied ovexwith bladder ifreciuired. As a sign of fine weather, the sediment of whiteflakes will settle near to the bottom of the tube, whilethe liquid will be quite transparent above. As a signof rain, the matter will rise to the surface of the so-lution. At the approach of a dorm, the matter wiUfloat on the surface of the solution in the form ofwhite flakes, and the fluid will appear in a state offermentation. During froit, the solution will pre-sent a starry ap Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/the-physician-and-pharmaceutist-solution-camphor-3-ij-jstitre-3-iss-sal-ammoniac-3-j-proof-spirit-i-ij3-solve-the-tube-maj-be-tied-ovexwith-bladder-ifreciuired-as-a-sign-of-fine-weather-the-sediment-of-whiteflakes-will-settle-near-to-the-bottom-of-the-tube-whilethe-liquid-will-be-quite-transparent-above-as-a-signof-rain-the-matter-will-rise-to-the-surface-of-the-so-lution-at-the-approach-of-a-dorm-the-matter-wiufloat-on-the-surface-of-the-solution-in-the-form-ofwhite-flakes-and-the-fluid-will-appear-in-a-state-offermentation-during-froit-the-solution-will-pre-sent-a-starry-ap-image338222956.htmlRM2AJ7AR8–The Physician and pharmaceutist . solution : Camphor 3 ij jSTitre 3 iss Sal Ammoniac 3 j Proof Spirit I ij3^. Solve. The tube maj^ be tied ovexwith bladder ifreciuired. As a sign of fine weather, the sediment of whiteflakes will settle near to the bottom of the tube, whilethe liquid will be quite transparent above. As a signof rain, the matter will rise to the surface of the so-lution. At the approach of a dorm, the matter wiUfloat on the surface of the solution in the form ofwhite flakes, and the fluid will appear in a state offermentation. During froit, the solution will pre-sent a starry ap
 . Electricity : its medical and surgical applications, including radiotherapy and phototherapy . Gravity cell. Sal ammoniac cell. The Sal Ammoniac Cell.—In this cell the exciting liquid is a solutionof sal ammoniac (NHiCl), the poles being carbon and zinc. As theliquid does not attack the zinc except when the current is flowing, thiscell does not deteriorate on standing, and it is therefore of use in so-called open circuit work, such as ringing bells, etc., where current isdrawn only occasionally. Sometimes the polarization is prevented bypacking the carbon pole in granulated peroxide of manga Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/electricity-its-medical-and-surgical-applications-including-radiotherapy-and-phototherapy-gravity-cell-sal-ammoniac-cell-the-sal-ammoniac-cellin-this-cell-the-exciting-liquid-is-a-solutionof-sal-ammoniac-nhicl-the-poles-being-carbon-and-zinc-as-theliquid-does-not-attack-the-zinc-except-when-the-current-is-flowing-thiscell-does-not-deteriorate-on-standing-and-it-is-therefore-of-use-in-so-called-open-circuit-work-such-as-ringing-bells-etc-where-current-isdrawn-only-occasionally-sometimes-the-polarization-is-prevented-bypacking-the-carbon-pole-in-granulated-peroxide-of-manga-image375981844.html
. Electricity : its medical and surgical applications, including radiotherapy and phototherapy . Gravity cell. Sal ammoniac cell. The Sal Ammoniac Cell.—In this cell the exciting liquid is a solutionof sal ammoniac (NHiCl), the poles being carbon and zinc. As theliquid does not attack the zinc except when the current is flowing, thiscell does not deteriorate on standing, and it is therefore of use in so-called open circuit work, such as ringing bells, etc., where current isdrawn only occasionally. Sometimes the polarization is prevented bypacking the carbon pole in granulated peroxide of manga Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/electricity-its-medical-and-surgical-applications-including-radiotherapy-and-phototherapy-gravity-cell-sal-ammoniac-cell-the-sal-ammoniac-cellin-this-cell-the-exciting-liquid-is-a-solutionof-sal-ammoniac-nhicl-the-poles-being-carbon-and-zinc-as-theliquid-does-not-attack-the-zinc-except-when-the-current-is-flowing-thiscell-does-not-deteriorate-on-standing-and-it-is-therefore-of-use-in-so-called-open-circuit-work-such-as-ringing-bells-etc-where-current-isdrawn-only-occasionally-sometimes-the-polarization-is-prevented-bypacking-the-carbon-pole-in-granulated-peroxide-of-manga-image375981844.htmlRM2CRKCK0–. Electricity : its medical and surgical applications, including radiotherapy and phototherapy . Gravity cell. Sal ammoniac cell. The Sal Ammoniac Cell.—In this cell the exciting liquid is a solutionof sal ammoniac (NHiCl), the poles being carbon and zinc. As theliquid does not attack the zinc except when the current is flowing, thiscell does not deteriorate on standing, and it is therefore of use in so-called open circuit work, such as ringing bells, etc., where current isdrawn only occasionally. Sometimes the polarization is prevented bypacking the carbon pole in granulated peroxide of manga
 . Electric railway gazette . is, so that the subsequentdiscussion of the matter will be easy. Such phenomena asdo not directly apply to the object of this series, will bestrictly avoided. The first thing that catches the eye when a railway motoris opened are the coils of wire. It is fair to assume thatthey are not put there for ornament, and that electric cur-rents go through them, and, therefore, it would be wiseto investigate just what the effect of coils of wire carryingcurrents is. To this end procure two magnets and two or more sal-ammoniac batteries from a dealer in electrical supplies, Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/electric-railway-gazette-is-so-that-the-subsequentdiscussion-of-the-matter-will-be-easy-such-phenomena-asdo-not-directly-apply-to-the-object-of-this-series-will-bestrictly-avoided-the-first-thing-that-catches-the-eye-when-a-railway-motoris-opened-are-the-coils-of-wire-it-is-fair-to-assume-thatthey-are-not-put-there-for-ornament-and-that-electric-cur-rents-go-through-them-and-therefore-it-would-be-wiseto-investigate-just-what-the-effect-of-coils-of-wire-carryingcurrents-is-to-this-end-procure-two-magnets-and-two-or-more-sal-ammoniac-batteries-from-a-dealer-in-electrical-supplies-image375737236.html
. Electric railway gazette . is, so that the subsequentdiscussion of the matter will be easy. Such phenomena asdo not directly apply to the object of this series, will bestrictly avoided. The first thing that catches the eye when a railway motoris opened are the coils of wire. It is fair to assume thatthey are not put there for ornament, and that electric cur-rents go through them, and, therefore, it would be wiseto investigate just what the effect of coils of wire carryingcurrents is. To this end procure two magnets and two or more sal-ammoniac batteries from a dealer in electrical supplies, Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/electric-railway-gazette-is-so-that-the-subsequentdiscussion-of-the-matter-will-be-easy-such-phenomena-asdo-not-directly-apply-to-the-object-of-this-series-will-bestrictly-avoided-the-first-thing-that-catches-the-eye-when-a-railway-motoris-opened-are-the-coils-of-wire-it-is-fair-to-assume-thatthey-are-not-put-there-for-ornament-and-that-electric-cur-rents-go-through-them-and-therefore-it-would-be-wiseto-investigate-just-what-the-effect-of-coils-of-wire-carryingcurrents-is-to-this-end-procure-two-magnets-and-two-or-more-sal-ammoniac-batteries-from-a-dealer-in-electrical-supplies-image375737236.htmlRM2CR88K0–. Electric railway gazette . is, so that the subsequentdiscussion of the matter will be easy. Such phenomena asdo not directly apply to the object of this series, will bestrictly avoided. The first thing that catches the eye when a railway motoris opened are the coils of wire. It is fair to assume thatthey are not put there for ornament, and that electric cur-rents go through them, and, therefore, it would be wiseto investigate just what the effect of coils of wire carryingcurrents is. To this end procure two magnets and two or more sal-ammoniac batteries from a dealer in electrical supplies,
 . The railroad and engineering journal . rless batteriesinvented, the primary object is to destroy the hydrogenas fast as it is evolved. In the Le Clanche battery, shown in tig. i, the copperis replaced by a rod of carbon which is surrounded bypowdered binoxide of manganese, a substance rich inoxygen. The two are enclosed in a porous jar which, withthe zinc rod, stands in a solution of sal-ammoniac. Theoxygen in the bino.xide attacks the hydrogen, and, if elec-tricity is not generated too fast, it destroys the hydrogenas fast as it is evolved. If. however, electricity—andtherefore hydrogen—is Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/the-railroad-and-engineering-journal-rless-batteriesinvented-the-primary-object-is-to-destroy-the-hydrogenas-fast-as-it-is-evolved-in-the-le-clanche-battery-shown-in-tig-i-the-copperis-replaced-by-a-rod-of-carbon-which-is-surrounded-bypowdered-binoxide-of-manganese-a-substance-rich-inoxygen-the-two-are-enclosed-in-a-porous-jar-which-withthe-zinc-rod-stands-in-a-solution-of-sal-ammoniac-theoxygen-in-the-binoxide-attacks-the-hydrogen-and-if-elec-tricity-is-not-generated-too-fast-it-destroys-the-hydrogenas-fast-as-it-is-evolved-if-however-electricityandtherefore-hydrogenis-image371961849.html
. The railroad and engineering journal . rless batteriesinvented, the primary object is to destroy the hydrogenas fast as it is evolved. In the Le Clanche battery, shown in tig. i, the copperis replaced by a rod of carbon which is surrounded bypowdered binoxide of manganese, a substance rich inoxygen. The two are enclosed in a porous jar which, withthe zinc rod, stands in a solution of sal-ammoniac. Theoxygen in the bino.xide attacks the hydrogen, and, if elec-tricity is not generated too fast, it destroys the hydrogenas fast as it is evolved. If. however, electricity—andtherefore hydrogen—is Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/the-railroad-and-engineering-journal-rless-batteriesinvented-the-primary-object-is-to-destroy-the-hydrogenas-fast-as-it-is-evolved-in-the-le-clanche-battery-shown-in-tig-i-the-copperis-replaced-by-a-rod-of-carbon-which-is-surrounded-bypowdered-binoxide-of-manganese-a-substance-rich-inoxygen-the-two-are-enclosed-in-a-porous-jar-which-withthe-zinc-rod-stands-in-a-solution-of-sal-ammoniac-theoxygen-in-the-binoxide-attacks-the-hydrogen-and-if-elec-tricity-is-not-generated-too-fast-it-destroys-the-hydrogenas-fast-as-it-is-evolved-if-however-electricityandtherefore-hydrogenis-image371961849.htmlRM2CH493N–. The railroad and engineering journal . rless batteriesinvented, the primary object is to destroy the hydrogenas fast as it is evolved. In the Le Clanche battery, shown in tig. i, the copperis replaced by a rod of carbon which is surrounded bypowdered binoxide of manganese, a substance rich inoxygen. The two are enclosed in a porous jar which, withthe zinc rod, stands in a solution of sal-ammoniac. Theoxygen in the bino.xide attacks the hydrogen, and, if elec-tricity is not generated too fast, it destroys the hydrogenas fast as it is evolved. If. however, electricity—andtherefore hydrogen—is
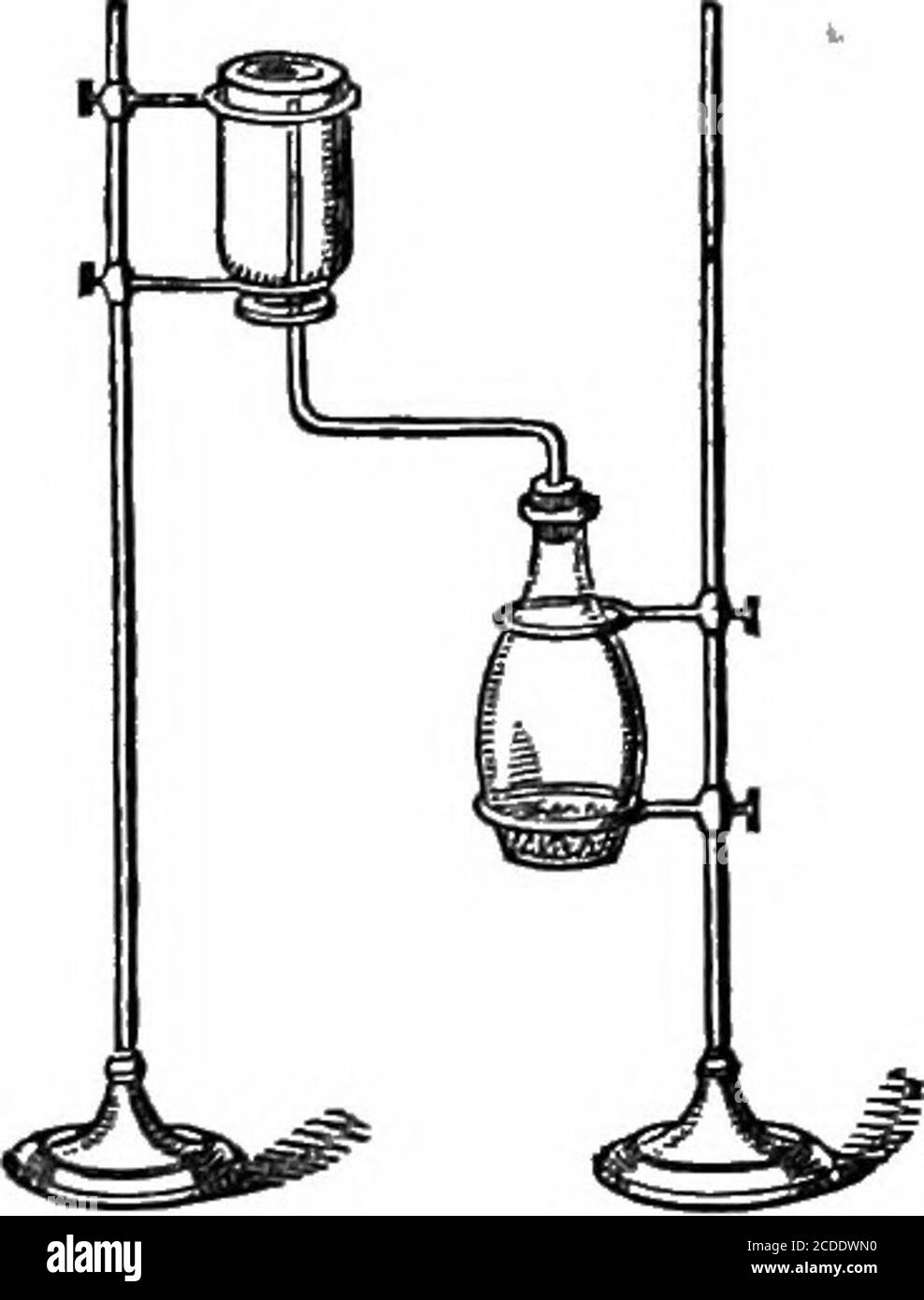 . Inorganic chemistry . eviously in union withthe alkali, and combines with the ammonia, forming the chlorideof anmionium (sal-ammoniac), NH^^Cl. This salt is purified byrepeated solution, crystallisation, and sublimation, and is thenready to yield ammonia. Metallic Elements. 229 759. The sal-ammoniac is reduced to powder, mixed withslaked quick-lime, and heated in aretort, or flask with a bent tube.Ammonia, in the state of gas, isevolved abundantly; and being verysoluble in water, must be collected atthe mercurial pneumatic trough, or bydisplacement of air. As it is onlyabout half as heavy as Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/inorganic-chemistry-eviously-in-union-withthe-alkali-and-combines-with-the-ammonia-forming-the-chlorideof-anmionium-sal-ammoniac-nhcl-this-salt-is-purified-byrepeated-solution-crystallisation-and-sublimation-and-is-thenready-to-yield-ammonia-metallic-elements-229-759-the-sal-ammoniac-is-reduced-to-powder-mixed-withslaked-quick-lime-and-heated-in-aretort-or-flask-with-a-bent-tubeammonia-in-the-state-of-gas-isevolved-abundantly-and-being-verysoluble-in-water-must-be-collected-atthe-mercurial-pneumatic-trough-or-bydisplacement-of-air-as-it-is-onlyabout-half-as-heavy-as-image369713820.html
. Inorganic chemistry . eviously in union withthe alkali, and combines with the ammonia, forming the chlorideof anmionium (sal-ammoniac), NH^^Cl. This salt is purified byrepeated solution, crystallisation, and sublimation, and is thenready to yield ammonia. Metallic Elements. 229 759. The sal-ammoniac is reduced to powder, mixed withslaked quick-lime, and heated in aretort, or flask with a bent tube.Ammonia, in the state of gas, isevolved abundantly; and being verysoluble in water, must be collected atthe mercurial pneumatic trough, or bydisplacement of air. As it is onlyabout half as heavy as Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/inorganic-chemistry-eviously-in-union-withthe-alkali-and-combines-with-the-ammonia-forming-the-chlorideof-anmionium-sal-ammoniac-nhcl-this-salt-is-purified-byrepeated-solution-crystallisation-and-sublimation-and-is-thenready-to-yield-ammonia-metallic-elements-229-759-the-sal-ammoniac-is-reduced-to-powder-mixed-withslaked-quick-lime-and-heated-in-aretort-or-flask-with-a-bent-tubeammonia-in-the-state-of-gas-isevolved-abundantly-and-being-verysoluble-in-water-must-be-collected-atthe-mercurial-pneumatic-trough-or-bydisplacement-of-air-as-it-is-onlyabout-half-as-heavy-as-image369713820.htmlRM2CDDWN0–. Inorganic chemistry . eviously in union withthe alkali, and combines with the ammonia, forming the chlorideof anmionium (sal-ammoniac), NH^^Cl. This salt is purified byrepeated solution, crystallisation, and sublimation, and is thenready to yield ammonia. Metallic Elements. 229 759. The sal-ammoniac is reduced to powder, mixed withslaked quick-lime, and heated in aretort, or flask with a bent tube.Ammonia, in the state of gas, isevolved abundantly; and being verysoluble in water, must be collected atthe mercurial pneumatic trough, or bydisplacement of air. As it is onlyabout half as heavy as
 . The practical telephone handbook and guide to the telephonic exchange . ig. 18 BA TTERIES 35 DRY CELLS.—Within the last few years various forms ofwhat are known as dry cells have come extensively into use.Those mostly used for telephonic purposes, such as the Helle-sen, the E.C.C., the Obach,the Delafon, etc., are allmodifications of the Leclanche cell, inasmuch as the same ele-ments, zinc and carbon ; the same depolariser, manganese diox-ide; and the same excitant, a solution of sal-ammoniac, are used.The term solution will seem strange in connection with a dry cell; but this term is a misn Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/the-practical-telephone-handbook-and-guide-to-the-telephonic-exchange-ig-18-ba-tteries-35-dry-cellswithin-the-last-few-years-various-forms-ofwhat-are-known-as-dry-cells-have-come-extensively-into-usethose-mostly-used-for-telephonic-purposes-such-as-the-helle-sen-the-ecc-the-obachthe-delafon-etc-are-allmodifications-of-the-leclanche-cell-inasmuch-as-the-same-ele-ments-zinc-and-carbon-the-same-depolariser-manganese-diox-ide-and-the-same-excitant-a-solution-of-sal-ammoniac-are-usedthe-term-solution-will-seem-strange-in-connection-with-a-dry-cell-but-this-term-is-a-misn-image374591050.html
. The practical telephone handbook and guide to the telephonic exchange . ig. 18 BA TTERIES 35 DRY CELLS.—Within the last few years various forms ofwhat are known as dry cells have come extensively into use.Those mostly used for telephonic purposes, such as the Helle-sen, the E.C.C., the Obach,the Delafon, etc., are allmodifications of the Leclanche cell, inasmuch as the same ele-ments, zinc and carbon ; the same depolariser, manganese diox-ide; and the same excitant, a solution of sal-ammoniac, are used.The term solution will seem strange in connection with a dry cell; but this term is a misn Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/the-practical-telephone-handbook-and-guide-to-the-telephonic-exchange-ig-18-ba-tteries-35-dry-cellswithin-the-last-few-years-various-forms-ofwhat-are-known-as-dry-cells-have-come-extensively-into-usethose-mostly-used-for-telephonic-purposes-such-as-the-helle-sen-the-ecc-the-obachthe-delafon-etc-are-allmodifications-of-the-leclanche-cell-inasmuch-as-the-same-ele-ments-zinc-and-carbon-the-same-depolariser-manganese-diox-ide-and-the-same-excitant-a-solution-of-sal-ammoniac-are-usedthe-term-solution-will-seem-strange-in-connection-with-a-dry-cell-but-this-term-is-a-misn-image374591050.htmlRM2CNC2KP–. The practical telephone handbook and guide to the telephonic exchange . ig. 18 BA TTERIES 35 DRY CELLS.—Within the last few years various forms ofwhat are known as dry cells have come extensively into use.Those mostly used for telephonic purposes, such as the Helle-sen, the E.C.C., the Obach,the Delafon, etc., are allmodifications of the Leclanche cell, inasmuch as the same ele-ments, zinc and carbon ; the same depolariser, manganese diox-ide; and the same excitant, a solution of sal-ammoniac, are used.The term solution will seem strange in connection with a dry cell; but this term is a misn
 . The practical telephone handbook and guide to the telephonic exchange . and the cell be-comes exhausted when thesolution of sal-ammoniac isused up. Most of the other formsof dry cell are similar tothis, differing only in detailsof the various pastes used.The internal resistance ofthese cells is much lowerthan that of the wet formof Leclanche, being whennew only about *1 of anohm; the cells will, there-fore, give a powerful currentthrough a small externalresistance. The E.M.F. isabout 1*4 volts per cell atfirst but rapidly falls when in action. Dry cells should bekept in a cool place, or they Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/the-practical-telephone-handbook-and-guide-to-the-telephonic-exchange-and-the-cell-be-comes-exhausted-when-thesolution-of-sal-ammoniac-isused-up-most-of-the-other-formsof-dry-cell-are-similar-tothis-differing-only-in-detailsof-the-various-pastes-usedthe-internal-resistance-ofthese-cells-is-much-lowerthan-that-of-the-wet-formof-leclanche-being-whennew-only-about-1-of-anohm-the-cells-will-there-fore-give-a-powerful-currentthrough-a-small-externalresistance-the-emf-isabout-14-volts-per-cell-atfirst-but-rapidly-falls-when-in-action-dry-cells-should-bekept-in-a-cool-place-or-they-image374591061.html
. The practical telephone handbook and guide to the telephonic exchange . and the cell be-comes exhausted when thesolution of sal-ammoniac isused up. Most of the other formsof dry cell are similar tothis, differing only in detailsof the various pastes used.The internal resistance ofthese cells is much lowerthan that of the wet formof Leclanche, being whennew only about *1 of anohm; the cells will, there-fore, give a powerful currentthrough a small externalresistance. The E.M.F. isabout 1*4 volts per cell atfirst but rapidly falls when in action. Dry cells should bekept in a cool place, or they Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/the-practical-telephone-handbook-and-guide-to-the-telephonic-exchange-and-the-cell-be-comes-exhausted-when-thesolution-of-sal-ammoniac-isused-up-most-of-the-other-formsof-dry-cell-are-similar-tothis-differing-only-in-detailsof-the-various-pastes-usedthe-internal-resistance-ofthese-cells-is-much-lowerthan-that-of-the-wet-formof-leclanche-being-whennew-only-about-1-of-anohm-the-cells-will-there-fore-give-a-powerful-currentthrough-a-small-externalresistance-the-emf-isabout-14-volts-per-cell-atfirst-but-rapidly-falls-when-in-action-dry-cells-should-bekept-in-a-cool-place-or-they-image374591061.htmlRM2CNC2M5–. The practical telephone handbook and guide to the telephonic exchange . and the cell be-comes exhausted when thesolution of sal-ammoniac isused up. Most of the other formsof dry cell are similar tothis, differing only in detailsof the various pastes used.The internal resistance ofthese cells is much lowerthan that of the wet formof Leclanche, being whennew only about *1 of anohm; the cells will, there-fore, give a powerful currentthrough a small externalresistance. The E.M.F. isabout 1*4 volts per cell atfirst but rapidly falls when in action. Dry cells should bekept in a cool place, or they
 . Illustrated catalogue and price list of bells, electric gas burners, batteries, push buttons ... . Cat. No. 500. Complete, . I 510. Carbon Cup and Connections, . 20 511. Jar, ^ 612. Zinc, ? 513. Sal-ammoniac, per lb., ..... 15 Cat. No. 1R K> 514. Complete, . $ 70 515. Porous Cup, ■ ■ . 50 516. Jar, 10 517. Zinc, 6 518. Sal Ammoniac, per 11)., 15 NOVELTY ELECTRIC COMPANY, The Gonda Cell The Axo Cell, No. J.IllustratedCatalogueAndPriceListOfBellsElectricGasBurnersBatteries. 5 Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/illustrated-catalogue-and-price-list-of-bells-electric-gas-burners-batteries-push-buttons-cat-no-500-complete-i-510-carbon-cup-and-connections-20-511-jar-612-zinc-513-sal-ammoniac-per-lb-15-cat-no-1r-kgt-514-complete-70-515-porous-cup-50-516-jar-10-517-zinc-6-518-sal-ammoniac-per-11-15-novelty-electric-company-the-gonda-cell-the-axo-cell-no-jillustratedcatalogueandpricelistofbellselectricgasburnersbatteries-5-image374610294.html
. Illustrated catalogue and price list of bells, electric gas burners, batteries, push buttons ... . Cat. No. 500. Complete, . I 510. Carbon Cup and Connections, . 20 511. Jar, ^ 612. Zinc, ? 513. Sal-ammoniac, per lb., ..... 15 Cat. No. 1R K> 514. Complete, . $ 70 515. Porous Cup, ■ ■ . 50 516. Jar, 10 517. Zinc, 6 518. Sal Ammoniac, per 11)., 15 NOVELTY ELECTRIC COMPANY, The Gonda Cell The Axo Cell, No. J.IllustratedCatalogueAndPriceListOfBellsElectricGasBurnersBatteries. 5 Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/illustrated-catalogue-and-price-list-of-bells-electric-gas-burners-batteries-push-buttons-cat-no-500-complete-i-510-carbon-cup-and-connections-20-511-jar-612-zinc-513-sal-ammoniac-per-lb-15-cat-no-1r-kgt-514-complete-70-515-porous-cup-50-516-jar-10-517-zinc-6-518-sal-ammoniac-per-11-15-novelty-electric-company-the-gonda-cell-the-axo-cell-no-jillustratedcatalogueandpricelistofbellselectricgasburnersbatteries-5-image374610294.htmlRM2CNCY72–. Illustrated catalogue and price list of bells, electric gas burners, batteries, push buttons ... . Cat. No. 500. Complete, . I 510. Carbon Cup and Connections, . 20 511. Jar, ^ 612. Zinc, ? 513. Sal-ammoniac, per lb., ..... 15 Cat. No. 1R K> 514. Complete, . $ 70 515. Porous Cup, ■ ■ . 50 516. Jar, 10 517. Zinc, 6 518. Sal Ammoniac, per 11)., 15 NOVELTY ELECTRIC COMPANY, The Gonda Cell The Axo Cell, No. J.IllustratedCatalogueAndPriceListOfBellsElectricGasBurnersBatteries. 5
 . Electrical instruments and telephones of the U.S. Signal corps . ^ of the cell. To place the cell in service, remove the. No.6RESERVE No.5RESERVE Fig. 4.—Reserve dry cells. No.4-0.RESERVE plug from the top of the carbon element and fill with water (rain waterpreferred). As soon as this is absorbed, fill again, and repeat until itwill take no more water, then throw out the excess water from thecarbon element, replacing the plug, and the cell is ready for use.When the cell becomes weak from use, a little sal-ammoniac solutionplaced inside the carbon element will rejuvenate the cell to someexte Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/electrical-instruments-and-telephones-of-the-us-signal-corps-of-the-cell-to-place-the-cell-in-service-remove-the-no6reserve-no5reserve-fig-4reserve-dry-cells-no4-0reserve-plug-from-the-top-of-the-carbon-element-and-fill-with-water-rain-waterpreferred-as-soon-as-this-is-absorbed-fill-again-and-repeat-until-itwill-take-no-more-water-then-throw-out-the-excess-water-from-thecarbon-element-replacing-the-plug-and-the-cell-is-ready-for-usewhen-the-cell-becomes-weak-from-use-a-little-sal-ammoniac-solutionplaced-inside-the-carbon-element-will-rejuvenate-the-cell-to-someexte-image376111856.html
. Electrical instruments and telephones of the U.S. Signal corps . ^ of the cell. To place the cell in service, remove the. No.6RESERVE No.5RESERVE Fig. 4.—Reserve dry cells. No.4-0.RESERVE plug from the top of the carbon element and fill with water (rain waterpreferred). As soon as this is absorbed, fill again, and repeat until itwill take no more water, then throw out the excess water from thecarbon element, replacing the plug, and the cell is ready for use.When the cell becomes weak from use, a little sal-ammoniac solutionplaced inside the carbon element will rejuvenate the cell to someexte Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/electrical-instruments-and-telephones-of-the-us-signal-corps-of-the-cell-to-place-the-cell-in-service-remove-the-no6reserve-no5reserve-fig-4reserve-dry-cells-no4-0reserve-plug-from-the-top-of-the-carbon-element-and-fill-with-water-rain-waterpreferred-as-soon-as-this-is-absorbed-fill-again-and-repeat-until-itwill-take-no-more-water-then-throw-out-the-excess-water-from-thecarbon-element-replacing-the-plug-and-the-cell-is-ready-for-usewhen-the-cell-becomes-weak-from-use-a-little-sal-ammoniac-solutionplaced-inside-the-carbon-element-will-rejuvenate-the-cell-to-someexte-image376111856.htmlRM2CRWAE8–. Electrical instruments and telephones of the U.S. Signal corps . ^ of the cell. To place the cell in service, remove the. No.6RESERVE No.5RESERVE Fig. 4.—Reserve dry cells. No.4-0.RESERVE plug from the top of the carbon element and fill with water (rain waterpreferred). As soon as this is absorbed, fill again, and repeat until itwill take no more water, then throw out the excess water from thecarbon element, replacing the plug, and the cell is ready for use.When the cell becomes weak from use, a little sal-ammoniac solutionplaced inside the carbon element will rejuvenate the cell to someexte
![. Illustrated catalogue and price list of bells, electric gas burners, batteries, push buttons ... . Cat. No •>;b. Complete, . .Zinc, . Jar 541. >4i Robbei to Suspend Carbon, .... Sal-ammoniac, . No. ■, ii:i. t> Cat. No.. $1 00 544. Complete, .>45. Carbon, . -546. Regular Zinc, r^7. Jar, . . 548. Cover, . .Sal-ammoniac 8a1815 PRI< F. |] 25 17 10 R I1 RTH - IllILAHEl IHI Gravity BatteryFor Closed Circuit Work. Cal No Eagle Metallic Battery Closed Circuit Stock Photo . Illustrated catalogue and price list of bells, electric gas burners, batteries, push buttons ... . Cat. No •>;b. Complete, . .Zinc, . Jar 541. >4i Robbei to Suspend Carbon, .... Sal-ammoniac, . No. ■, ii:i. t> Cat. No.. $1 00 544. Complete, .>45. Carbon, . -546. Regular Zinc, r^7. Jar, . . 548. Cover, . .Sal-ammoniac 8a1815 PRI< F. |] 25 17 10 R I1 RTH - IllILAHEl IHI Gravity BatteryFor Closed Circuit Work. Cal No Eagle Metallic Battery Closed Circuit Stock Photo](https://c8.alamy.com/comp/2CNCXG5/illustrated-catalogue-and-price-list-of-bells-electric-gas-burners-batteries-push-buttons-cat-no-gtb-complete-zinc-jar-541-gt4i-robbei-to-suspend-carbon-sal-ammoniac-no-iii-tgt-cat-no-1-00-544-complete-gt45-carbon-546-regular-zinc-r7-jar-548-cover-sal-ammoniac-8a1815-prilt-f-25-17-10-r-i1-rth-illilahel-ihi-gravity-batteryfor-closed-circuit-work-cal-no-eagle-metallic-battery-closed-circuit-2CNCXG5.jpg) . Illustrated catalogue and price list of bells, electric gas burners, batteries, push buttons ... . Cat. No •>;b. Complete, . .Zinc, . Jar 541. >4i Robbei to Suspend Carbon, .... Sal-ammoniac, . No. ■, ii:i. t> Cat. No.. $1 00 544. Complete, .>45. Carbon, . -546. Regular Zinc, r^7. Jar, . . 548. Cover, . .Sal-ammoniac 8a1815 PRI< F. |] 25 17 10 R I1 RTH - IllILAHEl IHI Gravity BatteryFor Closed Circuit Work. Cal No Eagle Metallic Battery Closed Circuit Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/illustrated-catalogue-and-price-list-of-bells-electric-gas-burners-batteries-push-buttons-cat-no-gtb-complete-zinc-jar-541-gt4i-robbei-to-suspend-carbon-sal-ammoniac-no-iii-tgt-cat-no-1-00-544-complete-gt45-carbon-546-regular-zinc-r7-jar-548-cover-sal-ammoniac-8a1815-prilt-f-25-17-10-r-i1-rth-illilahel-ihi-gravity-batteryfor-closed-circuit-work-cal-no-eagle-metallic-battery-closed-circuit-image374609765.html
. Illustrated catalogue and price list of bells, electric gas burners, batteries, push buttons ... . Cat. No •>;b. Complete, . .Zinc, . Jar 541. >4i Robbei to Suspend Carbon, .... Sal-ammoniac, . No. ■, ii:i. t> Cat. No.. $1 00 544. Complete, .>45. Carbon, . -546. Regular Zinc, r^7. Jar, . . 548. Cover, . .Sal-ammoniac 8a1815 PRI< F. |] 25 17 10 R I1 RTH - IllILAHEl IHI Gravity BatteryFor Closed Circuit Work. Cal No Eagle Metallic Battery Closed Circuit Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/illustrated-catalogue-and-price-list-of-bells-electric-gas-burners-batteries-push-buttons-cat-no-gtb-complete-zinc-jar-541-gt4i-robbei-to-suspend-carbon-sal-ammoniac-no-iii-tgt-cat-no-1-00-544-complete-gt45-carbon-546-regular-zinc-r7-jar-548-cover-sal-ammoniac-8a1815-prilt-f-25-17-10-r-i1-rth-illilahel-ihi-gravity-batteryfor-closed-circuit-work-cal-no-eagle-metallic-battery-closed-circuit-image374609765.htmlRM2CNCXG5–. Illustrated catalogue and price list of bells, electric gas burners, batteries, push buttons ... . Cat. No •>;b. Complete, . .Zinc, . Jar 541. >4i Robbei to Suspend Carbon, .... Sal-ammoniac, . No. ■, ii:i. t> Cat. No.. $1 00 544. Complete, .>45. Carbon, . -546. Regular Zinc, r^7. Jar, . . 548. Cover, . .Sal-ammoniac 8a1815 PRI< F. |] 25 17 10 R I1 RTH - IllILAHEl IHI Gravity BatteryFor Closed Circuit Work. Cal No Eagle Metallic Battery Closed Circuit
 . American telephone practice . : To set up, place not more than fourounces of prime white sal ammoniac in the jar. Fill the jar one- 90 AMERICAN TELEPHONE PRACTICE. third full of water and stir until the sal ammoniac is all dissolved.Then place the carbon and zinc elements in place. A little waterpoured in the vent-hole of the porous-pot forms will tend to hastenthe action. Unless a cell is subject to very severe use, it will requirebut little attention if it is a good one. Water should be added tosupply loss by evaporation. If the cell fails to work, examine itsterminals for poor connections Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/american-telephone-practice-to-set-up-place-not-more-than-fourounces-of-prime-white-sal-ammoniac-in-the-jar-fill-the-jar-one-90-american-telephone-practice-third-full-of-water-and-stir-until-the-sal-ammoniac-is-all-dissolvedthen-place-the-carbon-and-zinc-elements-in-place-a-little-waterpoured-in-the-vent-hole-of-the-porous-pot-forms-will-tend-to-hastenthe-action-unless-a-cell-is-subject-to-very-severe-use-it-will-requirebut-little-attention-if-it-is-a-good-one-water-should-be-added-tosupply-loss-by-evaporation-if-the-cell-fails-to-work-examine-itsterminals-for-poor-connections-image376134921.html
. American telephone practice . : To set up, place not more than fourounces of prime white sal ammoniac in the jar. Fill the jar one- 90 AMERICAN TELEPHONE PRACTICE. third full of water and stir until the sal ammoniac is all dissolved.Then place the carbon and zinc elements in place. A little waterpoured in the vent-hole of the porous-pot forms will tend to hastenthe action. Unless a cell is subject to very severe use, it will requirebut little attention if it is a good one. Water should be added tosupply loss by evaporation. If the cell fails to work, examine itsterminals for poor connections Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/american-telephone-practice-to-set-up-place-not-more-than-fourounces-of-prime-white-sal-ammoniac-in-the-jar-fill-the-jar-one-90-american-telephone-practice-third-full-of-water-and-stir-until-the-sal-ammoniac-is-all-dissolvedthen-place-the-carbon-and-zinc-elements-in-place-a-little-waterpoured-in-the-vent-hole-of-the-porous-pot-forms-will-tend-to-hastenthe-action-unless-a-cell-is-subject-to-very-severe-use-it-will-requirebut-little-attention-if-it-is-a-good-one-water-should-be-added-tosupply-loss-by-evaporation-if-the-cell-fails-to-work-examine-itsterminals-for-poor-connections-image376134921.htmlRM2CRXBX1–. American telephone practice . : To set up, place not more than fourounces of prime white sal ammoniac in the jar. Fill the jar one- 90 AMERICAN TELEPHONE PRACTICE. third full of water and stir until the sal ammoniac is all dissolved.Then place the carbon and zinc elements in place. A little waterpoured in the vent-hole of the porous-pot forms will tend to hastenthe action. Unless a cell is subject to very severe use, it will requirebut little attention if it is a good one. Water should be added tosupply loss by evaporation. If the cell fails to work, examine itsterminals for poor connections
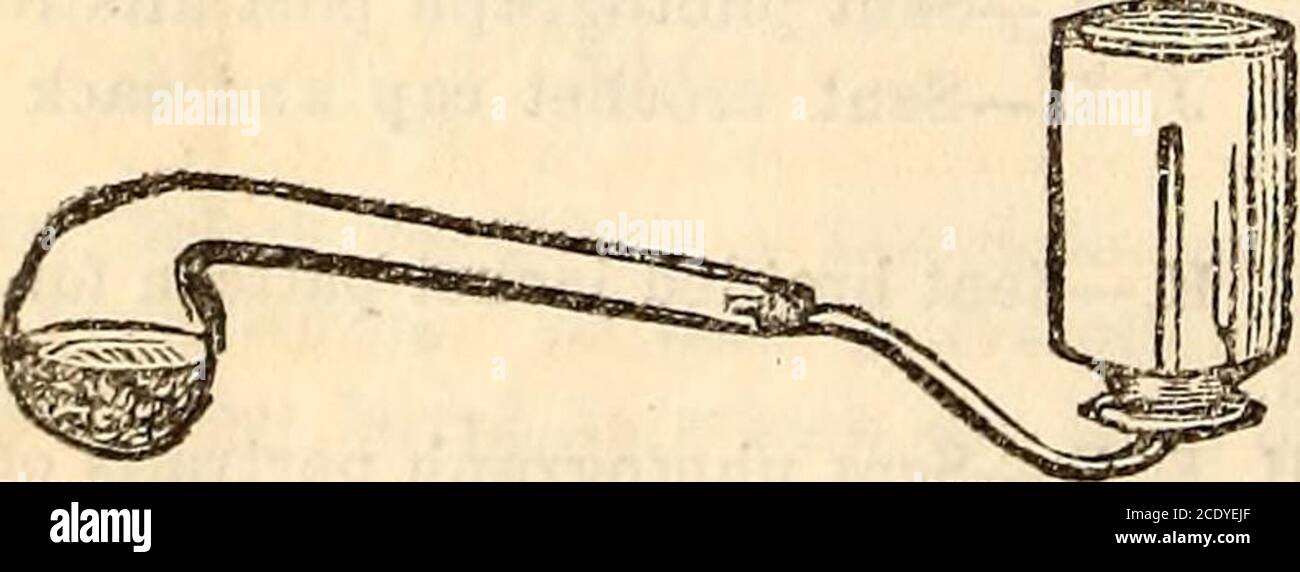 . Godey's lady's book . r-tight dishes, instead of pots. Petunias,Verbenas, and Fuchsias have rooted firmly by this me-thod. We shall give our experience in a subsequentnumber of the Ladys Book. (%ntisti|) for % frag. LESSON XX.—{Continued.)466. Ammonia.—Moisten some freshly-burned quick-lime with a little water. The lime will crumble topowder, or, in other words, become slacked. Mix equalparts of this slacked lime and sal-ammoniac. Put themixture into a small retort, and apply heat. A pungentodor will be recognizable ; from the evolution of am-monia in the form of gas. Ammonia being greedily Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/godeys-ladys-book-r-tight-dishes-instead-of-pots-petuniasverbenas-and-fuchsias-have-rooted-firmly-by-this-me-thod-we-shall-give-our-experience-in-a-subsequentnumber-of-the-ladys-book-ntisti-for-frag-lesson-xxcontinued466-ammoniamoisten-some-freshly-burned-quick-lime-with-a-little-water-the-lime-will-crumble-topowder-or-in-other-words-become-slacked-mix-equalparts-of-this-slacked-lime-and-sal-ammoniac-put-themixture-into-a-small-retort-and-apply-heat-a-pungentodor-will-be-recognizable-from-the-evolution-of-am-monia-in-the-form-of-gas-ammonia-being-greedily-image370012455.html
. Godey's lady's book . r-tight dishes, instead of pots. Petunias,Verbenas, and Fuchsias have rooted firmly by this me-thod. We shall give our experience in a subsequentnumber of the Ladys Book. (%ntisti|) for % frag. LESSON XX.—{Continued.)466. Ammonia.—Moisten some freshly-burned quick-lime with a little water. The lime will crumble topowder, or, in other words, become slacked. Mix equalparts of this slacked lime and sal-ammoniac. Put themixture into a small retort, and apply heat. A pungentodor will be recognizable ; from the evolution of am-monia in the form of gas. Ammonia being greedily Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/godeys-ladys-book-r-tight-dishes-instead-of-pots-petuniasverbenas-and-fuchsias-have-rooted-firmly-by-this-me-thod-we-shall-give-our-experience-in-a-subsequentnumber-of-the-ladys-book-ntisti-for-frag-lesson-xxcontinued466-ammoniamoisten-some-freshly-burned-quick-lime-with-a-little-water-the-lime-will-crumble-topowder-or-in-other-words-become-slacked-mix-equalparts-of-this-slacked-lime-and-sal-ammoniac-put-themixture-into-a-small-retort-and-apply-heat-a-pungentodor-will-be-recognizable-from-the-evolution-of-am-monia-in-the-form-of-gas-ammonia-being-greedily-image370012455.htmlRM2CDYEJF–. Godey's lady's book . r-tight dishes, instead of pots. Petunias,Verbenas, and Fuchsias have rooted firmly by this me-thod. We shall give our experience in a subsequentnumber of the Ladys Book. (%ntisti|) for % frag. LESSON XX.—{Continued.)466. Ammonia.—Moisten some freshly-burned quick-lime with a little water. The lime will crumble topowder, or, in other words, become slacked. Mix equalparts of this slacked lime and sal-ammoniac. Put themixture into a small retort, and apply heat. A pungentodor will be recognizable ; from the evolution of am-monia in the form of gas. Ammonia being greedily
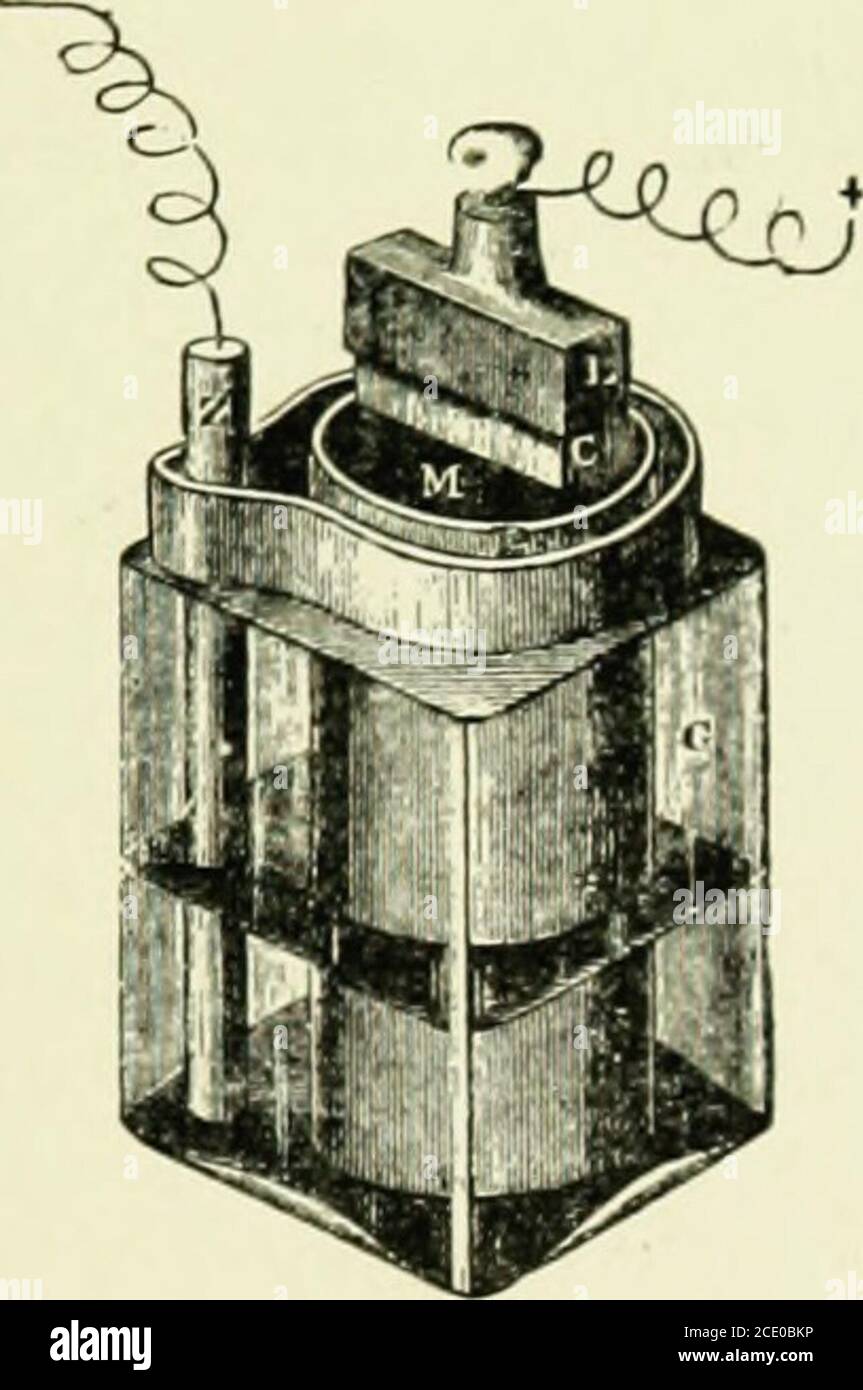 . Essentials of medical electricity . he Cell. This cell is in great demand when continuous use is not re-quired. It consists of a porous cup, in which is placed a car-bon plate, tightly packed in manganese peroxide and gas graph-ite ; a block of lead is soldered to the top of the carbon andattached thereto is a binding screw ; the cup is then immersedin a vessel one-third full of a strong solution of sal ammoniac ;the positive element consists of a rod of zinc having a copperwire attached. This cell develops an electro-motive force one-third greater than Daniels, and a resistance of from 2 to Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/essentials-of-medical-electricity-he-cell-this-cell-is-in-great-demand-when-continuous-use-is-not-re-quired-it-consists-of-a-porous-cup-in-which-is-placed-a-car-bon-plate-tightly-packed-in-manganese-peroxide-and-gas-graph-ite-a-block-of-lead-is-soldered-to-the-top-of-the-carbon-andattached-thereto-is-a-binding-screw-the-cup-is-then-immersedin-a-vessel-one-third-full-of-a-strong-solution-of-sal-ammoniac-the-positive-element-consists-of-a-rod-of-zinc-having-a-copperwire-attached-this-cell-develops-an-electro-motive-force-one-third-greater-than-daniels-and-a-resistance-of-from-2-to-image370032090.html
. Essentials of medical electricity . he Cell. This cell is in great demand when continuous use is not re-quired. It consists of a porous cup, in which is placed a car-bon plate, tightly packed in manganese peroxide and gas graph-ite ; a block of lead is soldered to the top of the carbon andattached thereto is a binding screw ; the cup is then immersedin a vessel one-third full of a strong solution of sal ammoniac ;the positive element consists of a rod of zinc having a copperwire attached. This cell develops an electro-motive force one-third greater than Daniels, and a resistance of from 2 to Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/essentials-of-medical-electricity-he-cell-this-cell-is-in-great-demand-when-continuous-use-is-not-re-quired-it-consists-of-a-porous-cup-in-which-is-placed-a-car-bon-plate-tightly-packed-in-manganese-peroxide-and-gas-graph-ite-a-block-of-lead-is-soldered-to-the-top-of-the-carbon-andattached-thereto-is-a-binding-screw-the-cup-is-then-immersedin-a-vessel-one-third-full-of-a-strong-solution-of-sal-ammoniac-the-positive-element-consists-of-a-rod-of-zinc-having-a-copperwire-attached-this-cell-develops-an-electro-motive-force-one-third-greater-than-daniels-and-a-resistance-of-from-2-to-image370032090.htmlRM2CE0BKP–. Essentials of medical electricity . he Cell. This cell is in great demand when continuous use is not re-quired. It consists of a porous cup, in which is placed a car-bon plate, tightly packed in manganese peroxide and gas graph-ite ; a block of lead is soldered to the top of the carbon andattached thereto is a binding screw ; the cup is then immersedin a vessel one-third full of a strong solution of sal ammoniac ;the positive element consists of a rod of zinc having a copperwire attached. This cell develops an electro-motive force one-third greater than Daniels, and a resistance of from 2 to
 . Agriculture and the farming business . utomatic sealing machines you will not needeither solder or additional heat. Simply buy caps and themetal rims and paper rings or gaskets with your cans. Forthe other sealing device use the standard rim-seal cans. Soldering equipment necessary.—Capping steel tip-ing copper, solder, sal ammoniac, a few scraps of zinc, twoand seven-sixteenths inches opening tipping, solder flux,a small brush, porcelain, glass or stone cup in whichto keep flux, a soft brick and a file. If using automaticsealer none of those things is needed. Soldering flux.—Soldering flux, Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/agriculture-and-the-farming-business-utomatic-sealing-machines-you-will-not-needeither-solder-or-additional-heat-simply-buy-caps-and-themetal-rims-and-paper-rings-or-gaskets-with-your-cans-forthe-other-sealing-device-use-the-standard-rim-seal-cans-soldering-equipment-necessarycapping-steel-tip-ing-copper-solder-sal-ammoniac-a-few-scraps-of-zinc-twoand-seven-sixteenths-inches-opening-tipping-solder-fluxa-small-brush-porcelain-glass-or-stone-cup-in-whichto-keep-flux-a-soft-brick-and-a-file-if-using-automaticsealer-none-of-those-things-is-needed-soldering-fluxsoldering-flux-image369849107.html
. Agriculture and the farming business . utomatic sealing machines you will not needeither solder or additional heat. Simply buy caps and themetal rims and paper rings or gaskets with your cans. Forthe other sealing device use the standard rim-seal cans. Soldering equipment necessary.—Capping steel tip-ing copper, solder, sal ammoniac, a few scraps of zinc, twoand seven-sixteenths inches opening tipping, solder flux,a small brush, porcelain, glass or stone cup in whichto keep flux, a soft brick and a file. If using automaticsealer none of those things is needed. Soldering flux.—Soldering flux, Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/agriculture-and-the-farming-business-utomatic-sealing-machines-you-will-not-needeither-solder-or-additional-heat-simply-buy-caps-and-themetal-rims-and-paper-rings-or-gaskets-with-your-cans-forthe-other-sealing-device-use-the-standard-rim-seal-cans-soldering-equipment-necessarycapping-steel-tip-ing-copper-solder-sal-ammoniac-a-few-scraps-of-zinc-twoand-seven-sixteenths-inches-opening-tipping-solder-fluxa-small-brush-porcelain-glass-or-stone-cup-in-whichto-keep-flux-a-soft-brick-and-a-file-if-using-automaticsealer-none-of-those-things-is-needed-soldering-fluxsoldering-flux-image369849107.htmlRM2CDM28K–. Agriculture and the farming business . utomatic sealing machines you will not needeither solder or additional heat. Simply buy caps and themetal rims and paper rings or gaskets with your cans. Forthe other sealing device use the standard rim-seal cans. Soldering equipment necessary.—Capping steel tip-ing copper, solder, sal ammoniac, a few scraps of zinc, twoand seven-sixteenths inches opening tipping, solder flux,a small brush, porcelain, glass or stone cup in whichto keep flux, a soft brick and a file. If using automaticsealer none of those things is needed. Soldering flux.—Soldering flux,
 . Electrical instruments and telephones of the U.S. Signal corps . No.6RESERVE No.5RESERVE Fig. 4.—Reserve dry cells. No.4-0.RESERVE plug from the top of the carbon element and fill with water (rain waterpreferred). As soon as this is absorbed, fill again, and repeat until itwill take no more water, then throw out the excess water from thecarbon element, replacing the plug, and the cell is ready for use.When the cell becomes weak from use, a little sal-ammoniac solutionplaced inside the carbon element will rejuvenate the cell to someextent. 24 ELECTRICAL INSTRUMENTS U. S. SIGNAL CORPS. DRY CEL Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/electrical-instruments-and-telephones-of-the-us-signal-corps-no6reserve-no5reserve-fig-4reserve-dry-cells-no4-0reserve-plug-from-the-top-of-the-carbon-element-and-fill-with-water-rain-waterpreferred-as-soon-as-this-is-absorbed-fill-again-and-repeat-until-itwill-take-no-more-water-then-throw-out-the-excess-water-from-thecarbon-element-replacing-the-plug-and-the-cell-is-ready-for-usewhen-the-cell-becomes-weak-from-use-a-little-sal-ammoniac-solutionplaced-inside-the-carbon-element-will-rejuvenate-the-cell-to-someextent-24-electrical-instruments-u-s-signal-corps-dry-cel-image376111879.html
. Electrical instruments and telephones of the U.S. Signal corps . No.6RESERVE No.5RESERVE Fig. 4.—Reserve dry cells. No.4-0.RESERVE plug from the top of the carbon element and fill with water (rain waterpreferred). As soon as this is absorbed, fill again, and repeat until itwill take no more water, then throw out the excess water from thecarbon element, replacing the plug, and the cell is ready for use.When the cell becomes weak from use, a little sal-ammoniac solutionplaced inside the carbon element will rejuvenate the cell to someextent. 24 ELECTRICAL INSTRUMENTS U. S. SIGNAL CORPS. DRY CEL Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/electrical-instruments-and-telephones-of-the-us-signal-corps-no6reserve-no5reserve-fig-4reserve-dry-cells-no4-0reserve-plug-from-the-top-of-the-carbon-element-and-fill-with-water-rain-waterpreferred-as-soon-as-this-is-absorbed-fill-again-and-repeat-until-itwill-take-no-more-water-then-throw-out-the-excess-water-from-thecarbon-element-replacing-the-plug-and-the-cell-is-ready-for-usewhen-the-cell-becomes-weak-from-use-a-little-sal-ammoniac-solutionplaced-inside-the-carbon-element-will-rejuvenate-the-cell-to-someextent-24-electrical-instruments-u-s-signal-corps-dry-cel-image376111879.htmlRM2CRWAF3–. Electrical instruments and telephones of the U.S. Signal corps . No.6RESERVE No.5RESERVE Fig. 4.—Reserve dry cells. No.4-0.RESERVE plug from the top of the carbon element and fill with water (rain waterpreferred). As soon as this is absorbed, fill again, and repeat until itwill take no more water, then throw out the excess water from thecarbon element, replacing the plug, and the cell is ready for use.When the cell becomes weak from use, a little sal-ammoniac solutionplaced inside the carbon element will rejuvenate the cell to someextent. 24 ELECTRICAL INSTRUMENTS U. S. SIGNAL CORPS. DRY CEL
 . Shield and compressed air tunneling . Fig. 131.—Tools used in calking with sal ammoniac and iron filings thejoints of an iron-lined tunnel. (Courtesy of Pennsylvania Railroad.)1. 2-lb. hammer. 2. Narrow calking tool. 3. Medium calking tool. 4.Wide calking tool. 5. Cutting and cleaning tool. 6. Pusher for placing themixture in the groove. 191. Cleaning the Groove.—Whatever the form of the grooves,they are thoroughly scraped out with a special tool, cleaned withcotton waste and washed with a stream of water under a pressureof at least 50 lb. per square inch. 192. Illustrations of Process.—Figu Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/shield-and-compressed-air-tunneling-fig-131tools-used-in-calking-with-sal-ammoniac-and-iron-filings-thejoints-of-an-iron-lined-tunnel-courtesy-of-pennsylvania-railroad1-2-lb-hammer-2-narrow-calking-tool-3-medium-calking-tool-4wide-calking-tool-5-cutting-and-cleaning-tool-6-pusher-for-placing-themixture-in-the-groove-191-cleaning-the-groovewhatever-the-form-of-the-groovesthey-are-thoroughly-scraped-out-with-a-special-tool-cleaned-withcotton-waste-and-washed-with-a-stream-of-water-under-a-pressureof-at-least-50-lb-per-square-inch-192-illustrations-of-processfigu-image370119183.html
. Shield and compressed air tunneling . Fig. 131.—Tools used in calking with sal ammoniac and iron filings thejoints of an iron-lined tunnel. (Courtesy of Pennsylvania Railroad.)1. 2-lb. hammer. 2. Narrow calking tool. 3. Medium calking tool. 4.Wide calking tool. 5. Cutting and cleaning tool. 6. Pusher for placing themixture in the groove. 191. Cleaning the Groove.—Whatever the form of the grooves,they are thoroughly scraped out with a special tool, cleaned withcotton waste and washed with a stream of water under a pressureof at least 50 lb. per square inch. 192. Illustrations of Process.—Figu Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/shield-and-compressed-air-tunneling-fig-131tools-used-in-calking-with-sal-ammoniac-and-iron-filings-thejoints-of-an-iron-lined-tunnel-courtesy-of-pennsylvania-railroad1-2-lb-hammer-2-narrow-calking-tool-3-medium-calking-tool-4wide-calking-tool-5-cutting-and-cleaning-tool-6-pusher-for-placing-themixture-in-the-groove-191-cleaning-the-groovewhatever-the-form-of-the-groovesthey-are-thoroughly-scraped-out-with-a-special-tool-cleaned-withcotton-waste-and-washed-with-a-stream-of-water-under-a-pressureof-at-least-50-lb-per-square-inch-192-illustrations-of-processfigu-image370119183.htmlRM2CE4AP7–. Shield and compressed air tunneling . Fig. 131.—Tools used in calking with sal ammoniac and iron filings thejoints of an iron-lined tunnel. (Courtesy of Pennsylvania Railroad.)1. 2-lb. hammer. 2. Narrow calking tool. 3. Medium calking tool. 4.Wide calking tool. 5. Cutting and cleaning tool. 6. Pusher for placing themixture in the groove. 191. Cleaning the Groove.—Whatever the form of the grooves,they are thoroughly scraped out with a special tool, cleaned withcotton waste and washed with a stream of water under a pressureof at least 50 lb. per square inch. 192. Illustrations of Process.—Figu
![. Illustrated catalogue and price list of bells, electric gas burners, batteries, push buttons ... . ]- N0 II TY II KCTRIC COMPANY Prices of Permanent Parts Edison-Lalande Cells Cat t 11 l; i w /nd X I 10 I «i»7U 10 Hi inin THE -Q K SAL-AMMONIAC BATTERY Double Cylinder. : PRICE LIST 52 & 54 NORTH FOURTH STREET, PHILADELPHIA, PA. ZINCS Rod Zincs Cat. No. phk u 672. Square , each, ... 678. Round, each 87 i. Round r it h Lug < ast, ti Stock Photo . Illustrated catalogue and price list of bells, electric gas burners, batteries, push buttons ... . ]- N0 II TY II KCTRIC COMPANY Prices of Permanent Parts Edison-Lalande Cells Cat t 11 l; i w /nd X I 10 I «i»7U 10 Hi inin THE -Q K SAL-AMMONIAC BATTERY Double Cylinder. : PRICE LIST 52 & 54 NORTH FOURTH STREET, PHILADELPHIA, PA. ZINCS Rod Zincs Cat. No. phk u 672. Square , each, ... 678. Round, each 87 i. Round r it h Lug < ast, ti Stock Photo](https://c8.alamy.com/comp/2CNCWCD/illustrated-catalogue-and-price-list-of-bells-electric-gas-burners-batteries-push-buttons-n0-ii-ty-ii-kctric-company-prices-of-permanent-parts-edison-lalande-cells-cat-t-11-l-i-w-nd-x-i-10-i-i7u-10-hi-inin-the-q-k-sal-ammoniac-battery-double-cylinder-price-list-52-54-north-fourth-street-philadelphia-pa-zincs-rod-zincs-cat-no-phk-u-672-square-each-678-round-each-87-i-round-r-it-h-lug-lt-ast-ti-2CNCWCD.jpg) . Illustrated catalogue and price list of bells, electric gas burners, batteries, push buttons ... . ]- N0 II TY II KCTRIC COMPANY Prices of Permanent Parts Edison-Lalande Cells Cat t 11 l; i w /nd X I 10 I «i»7U 10 Hi inin THE -Q K SAL-AMMONIAC BATTERY Double Cylinder. : PRICE LIST 52 & 54 NORTH FOURTH STREET, PHILADELPHIA, PA. ZINCS Rod Zincs Cat. No. phk u 672. Square , each, ... 678. Round, each 87 i. Round r it h Lug < ast, ti Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/illustrated-catalogue-and-price-list-of-bells-electric-gas-burners-batteries-push-buttons-n0-ii-ty-ii-kctric-company-prices-of-permanent-parts-edison-lalande-cells-cat-t-11-l-i-w-nd-x-i-10-i-i7u-10-hi-inin-the-q-k-sal-ammoniac-battery-double-cylinder-price-list-52-54-north-fourth-street-philadelphia-pa-zincs-rod-zincs-cat-no-phk-u-672-square-each-678-round-each-87-i-round-r-it-h-lug-lt-ast-ti-image374608877.html
. Illustrated catalogue and price list of bells, electric gas burners, batteries, push buttons ... . ]- N0 II TY II KCTRIC COMPANY Prices of Permanent Parts Edison-Lalande Cells Cat t 11 l; i w /nd X I 10 I «i»7U 10 Hi inin THE -Q K SAL-AMMONIAC BATTERY Double Cylinder. : PRICE LIST 52 & 54 NORTH FOURTH STREET, PHILADELPHIA, PA. ZINCS Rod Zincs Cat. No. phk u 672. Square , each, ... 678. Round, each 87 i. Round r it h Lug < ast, ti Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/illustrated-catalogue-and-price-list-of-bells-electric-gas-burners-batteries-push-buttons-n0-ii-ty-ii-kctric-company-prices-of-permanent-parts-edison-lalande-cells-cat-t-11-l-i-w-nd-x-i-10-i-i7u-10-hi-inin-the-q-k-sal-ammoniac-battery-double-cylinder-price-list-52-54-north-fourth-street-philadelphia-pa-zincs-rod-zincs-cat-no-phk-u-672-square-each-678-round-each-87-i-round-r-it-h-lug-lt-ast-ti-image374608877.htmlRM2CNCWCD–. Illustrated catalogue and price list of bells, electric gas burners, batteries, push buttons ... . ]- N0 II TY II KCTRIC COMPANY Prices of Permanent Parts Edison-Lalande Cells Cat t 11 l; i w /nd X I 10 I «i»7U 10 Hi inin THE -Q K SAL-AMMONIAC BATTERY Double Cylinder. : PRICE LIST 52 & 54 NORTH FOURTH STREET, PHILADELPHIA, PA. ZINCS Rod Zincs Cat. No. phk u 672. Square , each, ... 678. Round, each 87 i. Round r it h Lug < ast, ti
![. St. Nicholas [serial] . the wire will fit; then the elementsshould be clamped in between two pieces ofwood and held with screws, as shown in Fig. i.Another arrangement is shown in Fig. 2,where a zinc rod is suspended between twocarbons, the carbons being connected by a wirethat must not touch the zinc. A fruit-jar or a wide-necked pickle-bottle can be employed for a cell;but before the solution is poured in, the upperedge of the glass should be coated with paraf-fin. The solution is made by dissolving fourounces of sal-ammoniac in a pint of water, andthe jar should be filled three fourths fu Stock Photo . St. Nicholas [serial] . the wire will fit; then the elementsshould be clamped in between two pieces ofwood and held with screws, as shown in Fig. i.Another arrangement is shown in Fig. 2,where a zinc rod is suspended between twocarbons, the carbons being connected by a wirethat must not touch the zinc. A fruit-jar or a wide-necked pickle-bottle can be employed for a cell;but before the solution is poured in, the upperedge of the glass should be coated with paraf-fin. The solution is made by dissolving fourounces of sal-ammoniac in a pint of water, andthe jar should be filled three fourths fu Stock Photo](https://c8.alamy.com/comp/2CDCMRJ/st-nicholas-serial-the-wire-will-fit-then-the-elementsshould-be-clamped-in-between-two-pieces-ofwood-and-held-with-screws-as-shown-in-fig-ianother-arrangement-is-shown-in-fig-2where-a-zinc-rod-is-suspended-between-twocarbons-the-carbons-being-connected-by-a-wirethat-must-not-touch-the-zinc-a-fruit-jar-or-a-wide-necked-pickle-bottle-can-be-employed-for-a-cellbut-before-the-solution-is-poured-in-the-upperedge-of-the-glass-should-be-coated-with-paraf-fin-the-solution-is-made-by-dissolving-fourounces-of-sal-ammoniac-in-a-pint-of-water-andthe-jar-should-be-filled-three-fourths-fu-2CDCMRJ.jpg) . St. Nicholas [serial] . the wire will fit; then the elementsshould be clamped in between two pieces ofwood and held with screws, as shown in Fig. i.Another arrangement is shown in Fig. 2,where a zinc rod is suspended between twocarbons, the carbons being connected by a wirethat must not touch the zinc. A fruit-jar or a wide-necked pickle-bottle can be employed for a cell;but before the solution is poured in, the upperedge of the glass should be coated with paraf-fin. The solution is made by dissolving fourounces of sal-ammoniac in a pint of water, andthe jar should be filled three fourths fu Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/st-nicholas-serial-the-wire-will-fit-then-the-elementsshould-be-clamped-in-between-two-pieces-ofwood-and-held-with-screws-as-shown-in-fig-ianother-arrangement-is-shown-in-fig-2where-a-zinc-rod-is-suspended-between-twocarbons-the-carbons-being-connected-by-a-wirethat-must-not-touch-the-zinc-a-fruit-jar-or-a-wide-necked-pickle-bottle-can-be-employed-for-a-cellbut-before-the-solution-is-poured-in-the-upperedge-of-the-glass-should-be-coated-with-paraf-fin-the-solution-is-made-by-dissolving-fourounces-of-sal-ammoniac-in-a-pint-of-water-andthe-jar-should-be-filled-three-fourths-fu-image369688022.html
. St. Nicholas [serial] . the wire will fit; then the elementsshould be clamped in between two pieces ofwood and held with screws, as shown in Fig. i.Another arrangement is shown in Fig. 2,where a zinc rod is suspended between twocarbons, the carbons being connected by a wirethat must not touch the zinc. A fruit-jar or a wide-necked pickle-bottle can be employed for a cell;but before the solution is poured in, the upperedge of the glass should be coated with paraf-fin. The solution is made by dissolving fourounces of sal-ammoniac in a pint of water, andthe jar should be filled three fourths fu Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/st-nicholas-serial-the-wire-will-fit-then-the-elementsshould-be-clamped-in-between-two-pieces-ofwood-and-held-with-screws-as-shown-in-fig-ianother-arrangement-is-shown-in-fig-2where-a-zinc-rod-is-suspended-between-twocarbons-the-carbons-being-connected-by-a-wirethat-must-not-touch-the-zinc-a-fruit-jar-or-a-wide-necked-pickle-bottle-can-be-employed-for-a-cellbut-before-the-solution-is-poured-in-the-upperedge-of-the-glass-should-be-coated-with-paraf-fin-the-solution-is-made-by-dissolving-fourounces-of-sal-ammoniac-in-a-pint-of-water-andthe-jar-should-be-filled-three-fourths-fu-image369688022.htmlRM2CDCMRJ–. St. Nicholas [serial] . the wire will fit; then the elementsshould be clamped in between two pieces ofwood and held with screws, as shown in Fig. i.Another arrangement is shown in Fig. 2,where a zinc rod is suspended between twocarbons, the carbons being connected by a wirethat must not touch the zinc. A fruit-jar or a wide-necked pickle-bottle can be employed for a cell;but before the solution is poured in, the upperedge of the glass should be coated with paraf-fin. The solution is made by dissolving fourounces of sal-ammoniac in a pint of water, andthe jar should be filled three fourths fu
![. The miller, millwright and millfurnisher. ck toa blood-red heat ; then quench it in this mixture ; draw it gently over theclean fire till the spittle flashes off it ; then let it cool. 3. To 3 gallons ofwater add 3 ounces spirits of nitre, 3 ounces s])irits of hartshorn, 3 ounces POSITION IN DRESSING. 347 of white vitriol, 3 ounces of sal-ammoniac, 3 ounces of alum, 6 ounces ofsalt, with a double handful of hoof parings ; the steel should be heated adark cherry red. Used to temper picks for cutting French burr-stones. Another formula is as follows : i. Take 2 gallons rain-water, i ounce ofco Stock Photo . The miller, millwright and millfurnisher. ck toa blood-red heat ; then quench it in this mixture ; draw it gently over theclean fire till the spittle flashes off it ; then let it cool. 3. To 3 gallons ofwater add 3 ounces spirits of nitre, 3 ounces s])irits of hartshorn, 3 ounces POSITION IN DRESSING. 347 of white vitriol, 3 ounces of sal-ammoniac, 3 ounces of alum, 6 ounces ofsalt, with a double handful of hoof parings ; the steel should be heated adark cherry red. Used to temper picks for cutting French burr-stones. Another formula is as follows : i. Take 2 gallons rain-water, i ounce ofco Stock Photo](https://c8.alamy.com/comp/2CETJK5/the-miller-millwright-and-millfurnisher-ck-toa-blood-red-heat-then-quench-it-in-this-mixture-draw-it-gently-over-theclean-fire-till-the-spittle-flashes-off-it-then-let-it-cool-3-to-3-gallons-ofwater-add-3-ounces-spirits-of-nitre-3-ounces-s-irits-of-hartshorn-3-ounces-position-in-dressing-347-of-white-vitriol-3-ounces-of-sal-ammoniac-3-ounces-of-alum-6-ounces-ofsalt-with-a-double-handful-of-hoof-parings-the-steel-should-be-heated-adark-cherry-red-used-to-temper-picks-for-cutting-french-burr-stones-another-formula-is-as-follows-i-take-2-gallons-rain-water-i-ounce-ofco-2CETJK5.jpg) . The miller, millwright and millfurnisher. ck toa blood-red heat ; then quench it in this mixture ; draw it gently over theclean fire till the spittle flashes off it ; then let it cool. 3. To 3 gallons ofwater add 3 ounces spirits of nitre, 3 ounces s])irits of hartshorn, 3 ounces POSITION IN DRESSING. 347 of white vitriol, 3 ounces of sal-ammoniac, 3 ounces of alum, 6 ounces ofsalt, with a double handful of hoof parings ; the steel should be heated adark cherry red. Used to temper picks for cutting French burr-stones. Another formula is as follows : i. Take 2 gallons rain-water, i ounce ofco Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/the-miller-millwright-and-millfurnisher-ck-toa-blood-red-heat-then-quench-it-in-this-mixture-draw-it-gently-over-theclean-fire-till-the-spittle-flashes-off-it-then-let-it-cool-3-to-3-gallons-ofwater-add-3-ounces-spirits-of-nitre-3-ounces-s-irits-of-hartshorn-3-ounces-position-in-dressing-347-of-white-vitriol-3-ounces-of-sal-ammoniac-3-ounces-of-alum-6-ounces-ofsalt-with-a-double-handful-of-hoof-parings-the-steel-should-be-heated-adark-cherry-red-used-to-temper-picks-for-cutting-french-burr-stones-another-formula-is-as-follows-i-take-2-gallons-rain-water-i-ounce-ofco-image370564409.html
. The miller, millwright and millfurnisher. ck toa blood-red heat ; then quench it in this mixture ; draw it gently over theclean fire till the spittle flashes off it ; then let it cool. 3. To 3 gallons ofwater add 3 ounces spirits of nitre, 3 ounces s])irits of hartshorn, 3 ounces POSITION IN DRESSING. 347 of white vitriol, 3 ounces of sal-ammoniac, 3 ounces of alum, 6 ounces ofsalt, with a double handful of hoof parings ; the steel should be heated adark cherry red. Used to temper picks for cutting French burr-stones. Another formula is as follows : i. Take 2 gallons rain-water, i ounce ofco Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/the-miller-millwright-and-millfurnisher-ck-toa-blood-red-heat-then-quench-it-in-this-mixture-draw-it-gently-over-theclean-fire-till-the-spittle-flashes-off-it-then-let-it-cool-3-to-3-gallons-ofwater-add-3-ounces-spirits-of-nitre-3-ounces-s-irits-of-hartshorn-3-ounces-position-in-dressing-347-of-white-vitriol-3-ounces-of-sal-ammoniac-3-ounces-of-alum-6-ounces-ofsalt-with-a-double-handful-of-hoof-parings-the-steel-should-be-heated-adark-cherry-red-used-to-temper-picks-for-cutting-french-burr-stones-another-formula-is-as-follows-i-take-2-gallons-rain-water-i-ounce-ofco-image370564409.htmlRM2CETJK5–. The miller, millwright and millfurnisher. ck toa blood-red heat ; then quench it in this mixture ; draw it gently over theclean fire till the spittle flashes off it ; then let it cool. 3. To 3 gallons ofwater add 3 ounces spirits of nitre, 3 ounces s])irits of hartshorn, 3 ounces POSITION IN DRESSING. 347 of white vitriol, 3 ounces of sal-ammoniac, 3 ounces of alum, 6 ounces ofsalt, with a double handful of hoof parings ; the steel should be heated adark cherry red. Used to temper picks for cutting French burr-stones. Another formula is as follows : i. Take 2 gallons rain-water, i ounce ofco
 . The book of the garden. Gardening. 156 HEATING AS APPLIED IN HOETICULTURE. of his method of making joints. That it was necessary that they should be perfectly air-tight, is obvious; hence the more care was required in packing them. We believe that his joints were what is called rust joints, already noticed, and consisted merely of a paste formed of iron filings or borings, obtained at the iron-works, mixed with sal-ammoniac and water : a collar of hempen cord loosely spun is first intro- duced, and when driven hard in, the rust is introduced a little at a time, and also packed as hard as pos Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/the-book-of-the-garden-gardening-156-heating-as-applied-in-hoeticulture-of-his-method-of-making-joints-that-it-was-necessary-that-they-should-be-perfectly-air-tight-is-obvious-hence-the-more-care-was-required-in-packing-them-we-believe-that-his-joints-were-what-is-called-rust-joints-already-noticed-and-consisted-merely-of-a-paste-formed-of-iron-filings-or-borings-obtained-at-the-iron-works-mixed-with-sal-ammoniac-and-water-a-collar-of-hempen-cord-loosely-spun-is-first-intro-duced-and-when-driven-hard-in-the-rust-is-introduced-a-little-at-a-time-and-also-packed-as-hard-as-pos-image234450419.html
. The book of the garden. Gardening. 156 HEATING AS APPLIED IN HOETICULTURE. of his method of making joints. That it was necessary that they should be perfectly air-tight, is obvious; hence the more care was required in packing them. We believe that his joints were what is called rust joints, already noticed, and consisted merely of a paste formed of iron filings or borings, obtained at the iron-works, mixed with sal-ammoniac and water : a collar of hempen cord loosely spun is first intro- duced, and when driven hard in, the rust is introduced a little at a time, and also packed as hard as pos Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/the-book-of-the-garden-gardening-156-heating-as-applied-in-hoeticulture-of-his-method-of-making-joints-that-it-was-necessary-that-they-should-be-perfectly-air-tight-is-obvious-hence-the-more-care-was-required-in-packing-them-we-believe-that-his-joints-were-what-is-called-rust-joints-already-noticed-and-consisted-merely-of-a-paste-formed-of-iron-filings-or-borings-obtained-at-the-iron-works-mixed-with-sal-ammoniac-and-water-a-collar-of-hempen-cord-loosely-spun-is-first-intro-duced-and-when-driven-hard-in-the-rust-is-introduced-a-little-at-a-time-and-also-packed-as-hard-as-pos-image234450419.htmlRMRHC3W7–. The book of the garden. Gardening. 156 HEATING AS APPLIED IN HOETICULTURE. of his method of making joints. That it was necessary that they should be perfectly air-tight, is obvious; hence the more care was required in packing them. We believe that his joints were what is called rust joints, already noticed, and consisted merely of a paste formed of iron filings or borings, obtained at the iron-works, mixed with sal-ammoniac and water : a collar of hempen cord loosely spun is first intro- duced, and when driven hard in, the rust is introduced a little at a time, and also packed as hard as pos
 . Chemistry for farmers. Agricultural chemistry. 28 AGRICULTURAL CHEMISTRY. It is extremely irritating to tlie respiratory passages, when breathed even in the smallest quantities. It forms a large proportion of common salt, (Chloride of Sodium,) and an equal proportion of sal ammoniac, (Chloride of Ammonia.) The proportion of chlorine in each of these salts is sixty to every hundred pounds. Chlorine gas is easily prepared by adding chlorohydric acid to the oxide of manganese in a retort, or gas bottle, and applying a gentle heat. It may be collected in small quantities for immediate use, in a Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/chemistry-for-farmers-agricultural-chemistry-28-agricultural-chemistry-it-is-extremely-irritating-to-tlie-respiratory-passages-when-breathed-even-in-the-smallest-quantities-it-forms-a-large-proportion-of-common-salt-chloride-of-sodium-and-an-equal-proportion-of-sal-ammoniac-chloride-of-ammonia-the-proportion-of-chlorine-in-each-of-these-salts-is-sixty-to-every-hundred-pounds-chlorine-gas-is-easily-prepared-by-adding-chlorohydric-acid-to-the-oxide-of-manganese-in-a-retort-or-gas-bottle-and-applying-a-gentle-heat-it-may-be-collected-in-small-quantities-for-immediate-use-in-a-image234989745.html
. Chemistry for farmers. Agricultural chemistry. 28 AGRICULTURAL CHEMISTRY. It is extremely irritating to tlie respiratory passages, when breathed even in the smallest quantities. It forms a large proportion of common salt, (Chloride of Sodium,) and an equal proportion of sal ammoniac, (Chloride of Ammonia.) The proportion of chlorine in each of these salts is sixty to every hundred pounds. Chlorine gas is easily prepared by adding chlorohydric acid to the oxide of manganese in a retort, or gas bottle, and applying a gentle heat. It may be collected in small quantities for immediate use, in a Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/chemistry-for-farmers-agricultural-chemistry-28-agricultural-chemistry-it-is-extremely-irritating-to-tlie-respiratory-passages-when-breathed-even-in-the-smallest-quantities-it-forms-a-large-proportion-of-common-salt-chloride-of-sodium-and-an-equal-proportion-of-sal-ammoniac-chloride-of-ammonia-the-proportion-of-chlorine-in-each-of-these-salts-is-sixty-to-every-hundred-pounds-chlorine-gas-is-easily-prepared-by-adding-chlorohydric-acid-to-the-oxide-of-manganese-in-a-retort-or-gas-bottle-and-applying-a-gentle-heat-it-may-be-collected-in-small-quantities-for-immediate-use-in-a-image234989745.htmlRMRJ8KPW–. Chemistry for farmers. Agricultural chemistry. 28 AGRICULTURAL CHEMISTRY. It is extremely irritating to tlie respiratory passages, when breathed even in the smallest quantities. It forms a large proportion of common salt, (Chloride of Sodium,) and an equal proportion of sal ammoniac, (Chloride of Ammonia.) The proportion of chlorine in each of these salts is sixty to every hundred pounds. Chlorine gas is easily prepared by adding chlorohydric acid to the oxide of manganese in a retort, or gas bottle, and applying a gentle heat. It may be collected in small quantities for immediate use, in a
 . The art of taming and educating the horse : a system that makes easy and practical the subjection of wild and vicious horses ... : the simplest, most humane and effective in the world : with details of management in the subjection of over forty representative vicious horses, and the story of the author's personal experience : together with chapters on feeding, stabling, shoeing .... Horses; Horses; Horses; CHR 1887; PRO Smith, James Somers, Jr. (donor). 960 DISEASES AND THEIE TEEATMENT. Flfxor Tendon,^- m. 2 ouncea nitre (saltpetre), 2 ounces sal-ammoniac, 4 ounces common salt, 1 pint spring Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/the-art-of-taming-and-educating-the-horse-a-system-that-makes-easy-and-practical-the-subjection-of-wild-and-vicious-horses-the-simplest-most-humane-and-effective-in-the-world-with-details-of-management-in-the-subjection-of-over-forty-representative-vicious-horses-and-the-story-of-the-authors-personal-experience-together-with-chapters-on-feeding-stabling-shoeing-horses-horses-horses-chr-1887-pro-smith-james-somers-jr-donor-960-diseases-and-theie-teeatment-flfxor-tendon-m-2-ouncea-nitre-saltpetre-2-ounces-sal-ammoniac-4-ounces-common-salt-1-pint-spring-image235450444.html
. The art of taming and educating the horse : a system that makes easy and practical the subjection of wild and vicious horses ... : the simplest, most humane and effective in the world : with details of management in the subjection of over forty representative vicious horses, and the story of the author's personal experience : together with chapters on feeding, stabling, shoeing .... Horses; Horses; Horses; CHR 1887; PRO Smith, James Somers, Jr. (donor). 960 DISEASES AND THEIE TEEATMENT. Flfxor Tendon,^- m. 2 ouncea nitre (saltpetre), 2 ounces sal-ammoniac, 4 ounces common salt, 1 pint spring Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/the-art-of-taming-and-educating-the-horse-a-system-that-makes-easy-and-practical-the-subjection-of-wild-and-vicious-horses-the-simplest-most-humane-and-effective-in-the-world-with-details-of-management-in-the-subjection-of-over-forty-representative-vicious-horses-and-the-story-of-the-authors-personal-experience-together-with-chapters-on-feeding-stabling-shoeing-horses-horses-horses-chr-1887-pro-smith-james-somers-jr-donor-960-diseases-and-theie-teeatment-flfxor-tendon-m-2-ouncea-nitre-saltpetre-2-ounces-sal-ammoniac-4-ounces-common-salt-1-pint-spring-image235450444.htmlRMRK1KCC–. The art of taming and educating the horse : a system that makes easy and practical the subjection of wild and vicious horses ... : the simplest, most humane and effective in the world : with details of management in the subjection of over forty representative vicious horses, and the story of the author's personal experience : together with chapters on feeding, stabling, shoeing .... Horses; Horses; Horses; CHR 1887; PRO Smith, James Somers, Jr. (donor). 960 DISEASES AND THEIE TEEATMENT. Flfxor Tendon,^- m. 2 ouncea nitre (saltpetre), 2 ounces sal-ammoniac, 4 ounces common salt, 1 pint spring
 . Canadian forest industries 1894-1896. Lumbering; Forests and forestry; Forest products; Wood-pulp industry; Wood-using industries. 14 THE; CJLTTJLIDJL LUMBERMAN December, 1896 CLEANING DIRTY BELTS. A correspondent of the Centralblatt fur die Textil Industrie, who complains that several belts are dirty from drop oil and dust and desires to know how to clean them, is told by that journal to first wash the belts with warm water and soap, using a sharp, stiff brush, and while still moist, to rub them with a solution of sal- ammoniac, which saponifies the oil in them. Immediately thereafter the b Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/canadian-forest-industries-1894-1896-lumbering-forests-and-forestry-forest-products-wood-pulp-industry-wood-using-industries-14-the-cjlttjlidjl-lumberman-december-1896-cleaning-dirty-belts-a-correspondent-of-the-centralblatt-fur-die-textil-industrie-who-complains-that-several-belts-are-dirty-from-drop-oil-and-dust-and-desires-to-know-how-to-clean-them-is-told-by-that-journal-to-first-wash-the-belts-with-warm-water-and-soap-using-a-sharp-stiff-brush-and-while-still-moist-to-rub-them-with-a-solution-of-sal-ammoniac-which-saponifies-the-oil-in-them-immediately-thereafter-the-b-image233573050.html
. Canadian forest industries 1894-1896. Lumbering; Forests and forestry; Forest products; Wood-pulp industry; Wood-using industries. 14 THE; CJLTTJLIDJL LUMBERMAN December, 1896 CLEANING DIRTY BELTS. A correspondent of the Centralblatt fur die Textil Industrie, who complains that several belts are dirty from drop oil and dust and desires to know how to clean them, is told by that journal to first wash the belts with warm water and soap, using a sharp, stiff brush, and while still moist, to rub them with a solution of sal- ammoniac, which saponifies the oil in them. Immediately thereafter the b Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/canadian-forest-industries-1894-1896-lumbering-forests-and-forestry-forest-products-wood-pulp-industry-wood-using-industries-14-the-cjlttjlidjl-lumberman-december-1896-cleaning-dirty-belts-a-correspondent-of-the-centralblatt-fur-die-textil-industrie-who-complains-that-several-belts-are-dirty-from-drop-oil-and-dust-and-desires-to-know-how-to-clean-them-is-told-by-that-journal-to-first-wash-the-belts-with-warm-water-and-soap-using-a-sharp-stiff-brush-and-while-still-moist-to-rub-them-with-a-solution-of-sal-ammoniac-which-saponifies-the-oil-in-them-immediately-thereafter-the-b-image233573050.htmlRMRG04PJ–. Canadian forest industries 1894-1896. Lumbering; Forests and forestry; Forest products; Wood-pulp industry; Wood-using industries. 14 THE; CJLTTJLIDJL LUMBERMAN December, 1896 CLEANING DIRTY BELTS. A correspondent of the Centralblatt fur die Textil Industrie, who complains that several belts are dirty from drop oil and dust and desires to know how to clean them, is told by that journal to first wash the belts with warm water and soap, using a sharp, stiff brush, and while still moist, to rub them with a solution of sal- ammoniac, which saponifies the oil in them. Immediately thereafter the b
 . American bee journal. Bee culture; Bees. 342 AMERICAN BEE JOURNAL October much longer to solder straps on pails than it does to solder the cover 3 or 4 places in the rim, but I believe it is worth while. The average beekeeper who ships his honey in pails could sol- der all his pails for shipment in a short time, after he learns how. My soldering outfit consists of the following: A small trough for hold- ing the pails, two soldering irons, bar of solder, kerosene stove (kitchen stove), a pint earthen jar containing a weak solution of sal ammoniac, bot- tle of 'Ruby flux" and a mucilage b Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/american-bee-journal-bee-culture-bees-342-american-bee-journal-october-much-longer-to-solder-straps-on-pails-than-it-does-to-solder-the-cover-3-or-4-places-in-the-rim-but-i-believe-it-is-worth-while-the-average-beekeeper-who-ships-his-honey-in-pails-could-sol-der-all-his-pails-for-shipment-in-a-short-time-after-he-learns-how-my-soldering-outfit-consists-of-the-following-a-small-trough-for-hold-ing-the-pails-two-soldering-irons-bar-of-solder-kerosene-stove-kitchen-stove-a-pint-earthen-jar-containing-a-weak-solution-of-sal-ammoniac-bot-tle-of-ruby-fluxquot-and-a-mucilage-b-image237696514.html
. American bee journal. Bee culture; Bees. 342 AMERICAN BEE JOURNAL October much longer to solder straps on pails than it does to solder the cover 3 or 4 places in the rim, but I believe it is worth while. The average beekeeper who ships his honey in pails could sol- der all his pails for shipment in a short time, after he learns how. My soldering outfit consists of the following: A small trough for hold- ing the pails, two soldering irons, bar of solder, kerosene stove (kitchen stove), a pint earthen jar containing a weak solution of sal ammoniac, bot- tle of 'Ruby flux" and a mucilage b Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/american-bee-journal-bee-culture-bees-342-american-bee-journal-october-much-longer-to-solder-straps-on-pails-than-it-does-to-solder-the-cover-3-or-4-places-in-the-rim-but-i-believe-it-is-worth-while-the-average-beekeeper-who-ships-his-honey-in-pails-could-sol-der-all-his-pails-for-shipment-in-a-short-time-after-he-learns-how-my-soldering-outfit-consists-of-the-following-a-small-trough-for-hold-ing-the-pails-two-soldering-irons-bar-of-solder-kerosene-stove-kitchen-stove-a-pint-earthen-jar-containing-a-weak-solution-of-sal-ammoniac-bot-tle-of-ruby-fluxquot-and-a-mucilage-b-image237696514.htmlRMRPM096–. American bee journal. Bee culture; Bees. 342 AMERICAN BEE JOURNAL October much longer to solder straps on pails than it does to solder the cover 3 or 4 places in the rim, but I believe it is worth while. The average beekeeper who ships his honey in pails could sol- der all his pails for shipment in a short time, after he learns how. My soldering outfit consists of the following: A small trough for hold- ing the pails, two soldering irons, bar of solder, kerosene stove (kitchen stove), a pint earthen jar containing a weak solution of sal ammoniac, bot- tle of 'Ruby flux" and a mucilage b
 . Bulletin. Science. NO. I NEW STYLE SAMSON BATTERY. Cat. No. .,'^'<=«- 12369. Cell complete *1 06 PAETS: 14369. Carbon Vase i^H 16369. Cvlindrical Zinc 18 18369. Glass .lar, 7x85^ in. (new style) 6l^ 22369. RubberCover 09 24369. KiililxT Ring 06W 25369. Kubbev Plugs (per set of three) 07^ 26369. Sal Ammoniac 08 Cat. No. 12369. Cut illustrates New Style Carbon Vase, Zinc and Cover.. Please note that these images are extracted from scanned page images that may have been digitally enhanced for readability - coloration and appearance of these illustrations may not perfectly resemble the origi Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/bulletin-science-no-i-new-style-samson-battery-cat-no-lt=-12369-cell-complete-1-06-paets-14369-carbon-vase-ih-16369-cvlindrical-zinc-18-18369-glass-lar-7x85-in-new-style-6l-22369-rubbercover-09-24369-kiililxt-ring-06w-25369-kubbev-plugs-per-set-of-three-07-26369-sal-ammoniac-08-cat-no-12369-cut-illustrates-new-style-carbon-vase-zinc-and-cover-please-note-that-these-images-are-extracted-from-scanned-page-images-that-may-have-been-digitally-enhanced-for-readability-coloration-and-appearance-of-these-illustrations-may-not-perfectly-resemble-the-origi-image234187998.html
. Bulletin. Science. NO. I NEW STYLE SAMSON BATTERY. Cat. No. .,'^'<=«- 12369. Cell complete *1 06 PAETS: 14369. Carbon Vase i^H 16369. Cvlindrical Zinc 18 18369. Glass .lar, 7x85^ in. (new style) 6l^ 22369. RubberCover 09 24369. KiililxT Ring 06W 25369. Kubbev Plugs (per set of three) 07^ 26369. Sal Ammoniac 08 Cat. No. 12369. Cut illustrates New Style Carbon Vase, Zinc and Cover.. Please note that these images are extracted from scanned page images that may have been digitally enhanced for readability - coloration and appearance of these illustrations may not perfectly resemble the origi Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/bulletin-science-no-i-new-style-samson-battery-cat-no-lt=-12369-cell-complete-1-06-paets-14369-carbon-vase-ih-16369-cvlindrical-zinc-18-18369-glass-lar-7x85-in-new-style-6l-22369-rubbercover-09-24369-kiililxt-ring-06w-25369-kubbev-plugs-per-set-of-three-07-26369-sal-ammoniac-08-cat-no-12369-cut-illustrates-new-style-carbon-vase-zinc-and-cover-please-note-that-these-images-are-extracted-from-scanned-page-images-that-may-have-been-digitally-enhanced-for-readability-coloration-and-appearance-of-these-illustrations-may-not-perfectly-resemble-the-origi-image234187998.htmlRMRH0552–. Bulletin. Science. NO. I NEW STYLE SAMSON BATTERY. Cat. No. .,'^'<=«- 12369. Cell complete *1 06 PAETS: 14369. Carbon Vase i^H 16369. Cvlindrical Zinc 18 18369. Glass .lar, 7x85^ in. (new style) 6l^ 22369. RubberCover 09 24369. KiililxT Ring 06W 25369. Kubbev Plugs (per set of three) 07^ 26369. Sal Ammoniac 08 Cat. No. 12369. Cut illustrates New Style Carbon Vase, Zinc and Cover.. Please note that these images are extracted from scanned page images that may have been digitally enhanced for readability - coloration and appearance of these illustrations may not perfectly resemble the origi
 . Bulletin. Science. SHEET No. 369. THE E. S. GREELEY & CO., (^.t^^l'ZVico.) NEW YORK.. NO. I NEW STYLE SAMSON BATTERY. Cat. No. .,'^'<=«- 12369. Cell complete *1 06 PAETS: 14369. Carbon Vase i^H 16369. Cvlindrical Zinc 18 18369. Glass .lar, 7x85^ in. (new style) 6l^ 22369. RubberCover 09 24369. KiililxT Ring 06W 25369. Kubbev Plugs (per set of three) 07^ 26369. Sal Ammoniac 08 Cat. No. 12369. Cut illustrates New Style Carbon Vase, Zinc and Cover.. Please note that these images are extracted from scanned page images that may have been digitally enhanced for readability - coloration and Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/bulletin-science-sheet-no-369-the-e-s-greeley-amp-co-tlzvico-new-york-no-i-new-style-samson-battery-cat-no-lt=-12369-cell-complete-1-06-paets-14369-carbon-vase-ih-16369-cvlindrical-zinc-18-18369-glass-lar-7x85-in-new-style-6l-22369-rubbercover-09-24369-kiililxt-ring-06w-25369-kubbev-plugs-per-set-of-three-07-26369-sal-ammoniac-08-cat-no-12369-cut-illustrates-new-style-carbon-vase-zinc-and-cover-please-note-that-these-images-are-extracted-from-scanned-page-images-that-may-have-been-digitally-enhanced-for-readability-coloration-and-image234188010.html
. Bulletin. Science. SHEET No. 369. THE E. S. GREELEY & CO., (^.t^^l'ZVico.) NEW YORK.. NO. I NEW STYLE SAMSON BATTERY. Cat. No. .,'^'<=«- 12369. Cell complete *1 06 PAETS: 14369. Carbon Vase i^H 16369. Cvlindrical Zinc 18 18369. Glass .lar, 7x85^ in. (new style) 6l^ 22369. RubberCover 09 24369. KiililxT Ring 06W 25369. Kubbev Plugs (per set of three) 07^ 26369. Sal Ammoniac 08 Cat. No. 12369. Cut illustrates New Style Carbon Vase, Zinc and Cover.. Please note that these images are extracted from scanned page images that may have been digitally enhanced for readability - coloration and Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/bulletin-science-sheet-no-369-the-e-s-greeley-amp-co-tlzvico-new-york-no-i-new-style-samson-battery-cat-no-lt=-12369-cell-complete-1-06-paets-14369-carbon-vase-ih-16369-cvlindrical-zinc-18-18369-glass-lar-7x85-in-new-style-6l-22369-rubbercover-09-24369-kiililxt-ring-06w-25369-kubbev-plugs-per-set-of-three-07-26369-sal-ammoniac-08-cat-no-12369-cut-illustrates-new-style-carbon-vase-zinc-and-cover-please-note-that-these-images-are-extracted-from-scanned-page-images-that-may-have-been-digitally-enhanced-for-readability-coloration-and-image234188010.htmlRMRH055E–. Bulletin. Science. SHEET No. 369. THE E. S. GREELEY & CO., (^.t^^l'ZVico.) NEW YORK.. NO. I NEW STYLE SAMSON BATTERY. Cat. No. .,'^'<=«- 12369. Cell complete *1 06 PAETS: 14369. Carbon Vase i^H 16369. Cvlindrical Zinc 18 18369. Glass .lar, 7x85^ in. (new style) 6l^ 22369. RubberCover 09 24369. KiililxT Ring 06W 25369. Kubbev Plugs (per set of three) 07^ 26369. Sal Ammoniac 08 Cat. No. 12369. Cut illustrates New Style Carbon Vase, Zinc and Cover.. Please note that these images are extracted from scanned page images that may have been digitally enhanced for readability - coloration and
 . De re metallica. Metallurgy; Mineral industries. BOOK X. 455 Those ingredients above are peculiar to each cement, but what follows is common to all. Each of the ingredients is first separately crushed to powder ; the bricks are placed on a hard rock or marble, and crushed with an iron implement ; the other things are crushed in a mortar with a pestle ; each is separately passed through a sieve. Then they arc all mixed together, and are moistened with vinegar in which a little sal-ammoniac has been dissolved, if the cement does not contain any. But some workers, however, prefer to moisten the Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/de-re-metallica-metallurgy-mineral-industries-book-x-455-those-ingredients-above-are-peculiar-to-each-cement-but-what-follows-is-common-to-all-each-of-the-ingredients-is-first-separately-crushed-to-powder-the-bricks-are-placed-on-a-hard-rock-or-marble-and-crushed-with-an-iron-implement-the-other-things-are-crushed-in-a-mortar-with-a-pestle-each-is-separately-passed-through-a-sieve-then-they-arc-all-mixed-together-and-are-moistened-with-vinegar-in-which-a-little-sal-ammoniac-has-been-dissolved-if-the-cement-does-not-contain-any-but-some-workers-however-prefer-to-moisten-the-image231712215.html
. De re metallica. Metallurgy; Mineral industries. BOOK X. 455 Those ingredients above are peculiar to each cement, but what follows is common to all. Each of the ingredients is first separately crushed to powder ; the bricks are placed on a hard rock or marble, and crushed with an iron implement ; the other things are crushed in a mortar with a pestle ; each is separately passed through a sieve. Then they arc all mixed together, and are moistened with vinegar in which a little sal-ammoniac has been dissolved, if the cement does not contain any. But some workers, however, prefer to moisten the Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/de-re-metallica-metallurgy-mineral-industries-book-x-455-those-ingredients-above-are-peculiar-to-each-cement-but-what-follows-is-common-to-all-each-of-the-ingredients-is-first-separately-crushed-to-powder-the-bricks-are-placed-on-a-hard-rock-or-marble-and-crushed-with-an-iron-implement-the-other-things-are-crushed-in-a-mortar-with-a-pestle-each-is-separately-passed-through-a-sieve-then-they-arc-all-mixed-together-and-are-moistened-with-vinegar-in-which-a-little-sal-ammoniac-has-been-dissolved-if-the-cement-does-not-contain-any-but-some-workers-however-prefer-to-moisten-the-image231712215.htmlRMRCYB87–. De re metallica. Metallurgy; Mineral industries. BOOK X. 455 Those ingredients above are peculiar to each cement, but what follows is common to all. Each of the ingredients is first separately crushed to powder ; the bricks are placed on a hard rock or marble, and crushed with an iron implement ; the other things are crushed in a mortar with a pestle ; each is separately passed through a sieve. Then they arc all mixed together, and are moistened with vinegar in which a little sal-ammoniac has been dissolved, if the cement does not contain any. But some workers, however, prefer to moisten the
![. The elements of physiological physics: an outline of the elementary facts, principles, and methods of physics; and their applications in physiology. Biophysics. Chap. II.] LECLANCHE'S ELEMENT. work with, owing to absence of fumes. It can, therefore, be allowed on the table at which one is at work. Carbon is -f pole, zinc —. L,eclaiielif»'s cell (Fig. 12) consists of an outer compartment, containing a zinc cylinder z in a solution of sal- ammoniac. The inner compart- ment is a porous cell T, filled with a mixture of powdered carbon and black oxide of manganese (pyrolusite) sur- rounding a pla Stock Photo . The elements of physiological physics: an outline of the elementary facts, principles, and methods of physics; and their applications in physiology. Biophysics. Chap. II.] LECLANCHE'S ELEMENT. work with, owing to absence of fumes. It can, therefore, be allowed on the table at which one is at work. Carbon is -f pole, zinc —. L,eclaiielif»'s cell (Fig. 12) consists of an outer compartment, containing a zinc cylinder z in a solution of sal- ammoniac. The inner compart- ment is a porous cell T, filled with a mixture of powdered carbon and black oxide of manganese (pyrolusite) sur- rounding a pla Stock Photo](https://c8.alamy.com/comp/RCFN58/the-elements-of-physiological-physics-an-outline-of-the-elementary-facts-principles-and-methods-of-physics-and-their-applications-in-physiology-biophysics-chap-ii-leclanches-element-work-with-owing-to-absence-of-fumes-it-can-therefore-be-allowed-on-the-table-at-which-one-is-at-work-carbon-is-f-pole-zinc-leclaiielifs-cell-fig-12-consists-of-an-outer-compartment-containing-a-zinc-cylinder-z-in-a-solution-of-sal-ammoniac-the-inner-compart-ment-is-a-porous-cell-t-filled-with-a-mixture-of-powdered-carbon-and-black-oxide-of-manganese-pyrolusite-sur-rounding-a-pla-RCFN58.jpg) . The elements of physiological physics: an outline of the elementary facts, principles, and methods of physics; and their applications in physiology. Biophysics. Chap. II.] LECLANCHE'S ELEMENT. work with, owing to absence of fumes. It can, therefore, be allowed on the table at which one is at work. Carbon is -f pole, zinc —. L,eclaiielif»'s cell (Fig. 12) consists of an outer compartment, containing a zinc cylinder z in a solution of sal- ammoniac. The inner compart- ment is a porous cell T, filled with a mixture of powdered carbon and black oxide of manganese (pyrolusite) sur- rounding a pla Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/the-elements-of-physiological-physics-an-outline-of-the-elementary-facts-principles-and-methods-of-physics-and-their-applications-in-physiology-biophysics-chap-ii-leclanches-element-work-with-owing-to-absence-of-fumes-it-can-therefore-be-allowed-on-the-table-at-which-one-is-at-work-carbon-is-f-pole-zinc-leclaiielifs-cell-fig-12-consists-of-an-outer-compartment-containing-a-zinc-cylinder-z-in-a-solution-of-sal-ammoniac-the-inner-compart-ment-is-a-porous-cell-t-filled-with-a-mixture-of-powdered-carbon-and-black-oxide-of-manganese-pyrolusite-sur-rounding-a-pla-image231456548.html
. The elements of physiological physics: an outline of the elementary facts, principles, and methods of physics; and their applications in physiology. Biophysics. Chap. II.] LECLANCHE'S ELEMENT. work with, owing to absence of fumes. It can, therefore, be allowed on the table at which one is at work. Carbon is -f pole, zinc —. L,eclaiielif»'s cell (Fig. 12) consists of an outer compartment, containing a zinc cylinder z in a solution of sal- ammoniac. The inner compart- ment is a porous cell T, filled with a mixture of powdered carbon and black oxide of manganese (pyrolusite) sur- rounding a pla Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/the-elements-of-physiological-physics-an-outline-of-the-elementary-facts-principles-and-methods-of-physics-and-their-applications-in-physiology-biophysics-chap-ii-leclanches-element-work-with-owing-to-absence-of-fumes-it-can-therefore-be-allowed-on-the-table-at-which-one-is-at-work-carbon-is-f-pole-zinc-leclaiielifs-cell-fig-12-consists-of-an-outer-compartment-containing-a-zinc-cylinder-z-in-a-solution-of-sal-ammoniac-the-inner-compart-ment-is-a-porous-cell-t-filled-with-a-mixture-of-powdered-carbon-and-black-oxide-of-manganese-pyrolusite-sur-rounding-a-pla-image231456548.htmlRMRCFN58–. The elements of physiological physics: an outline of the elementary facts, principles, and methods of physics; and their applications in physiology. Biophysics. Chap. II.] LECLANCHE'S ELEMENT. work with, owing to absence of fumes. It can, therefore, be allowed on the table at which one is at work. Carbon is -f pole, zinc —. L,eclaiielif»'s cell (Fig. 12) consists of an outer compartment, containing a zinc cylinder z in a solution of sal- ammoniac. The inner compart- ment is a porous cell T, filled with a mixture of powdered carbon and black oxide of manganese (pyrolusite) sur- rounding a pla