Thermodynamics heat flow Stock Photos and Images
 A side view of a plate heat exchanger assembly, highlighting the arrangement of plates used for effective heat transfer in various applications. Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/a-side-view-of-a-plate-heat-exchanger-assembly-highlighting-the-arrangement-of-plates-used-for-effective-heat-transfer-in-various-applications-image617434482.html
A side view of a plate heat exchanger assembly, highlighting the arrangement of plates used for effective heat transfer in various applications. Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/a-side-view-of-a-plate-heat-exchanger-assembly-highlighting-the-arrangement-of-plates-used-for-effective-heat-transfer-in-various-applications-image617434482.htmlRF2XTEFYE–A side view of a plate heat exchanger assembly, highlighting the arrangement of plates used for effective heat transfer in various applications.
 Air Conditioner Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/stock-image-air-conditioner-160367800.html
Air Conditioner Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/stock-image-air-conditioner-160367800.htmlRFK8WANC–Air Conditioner
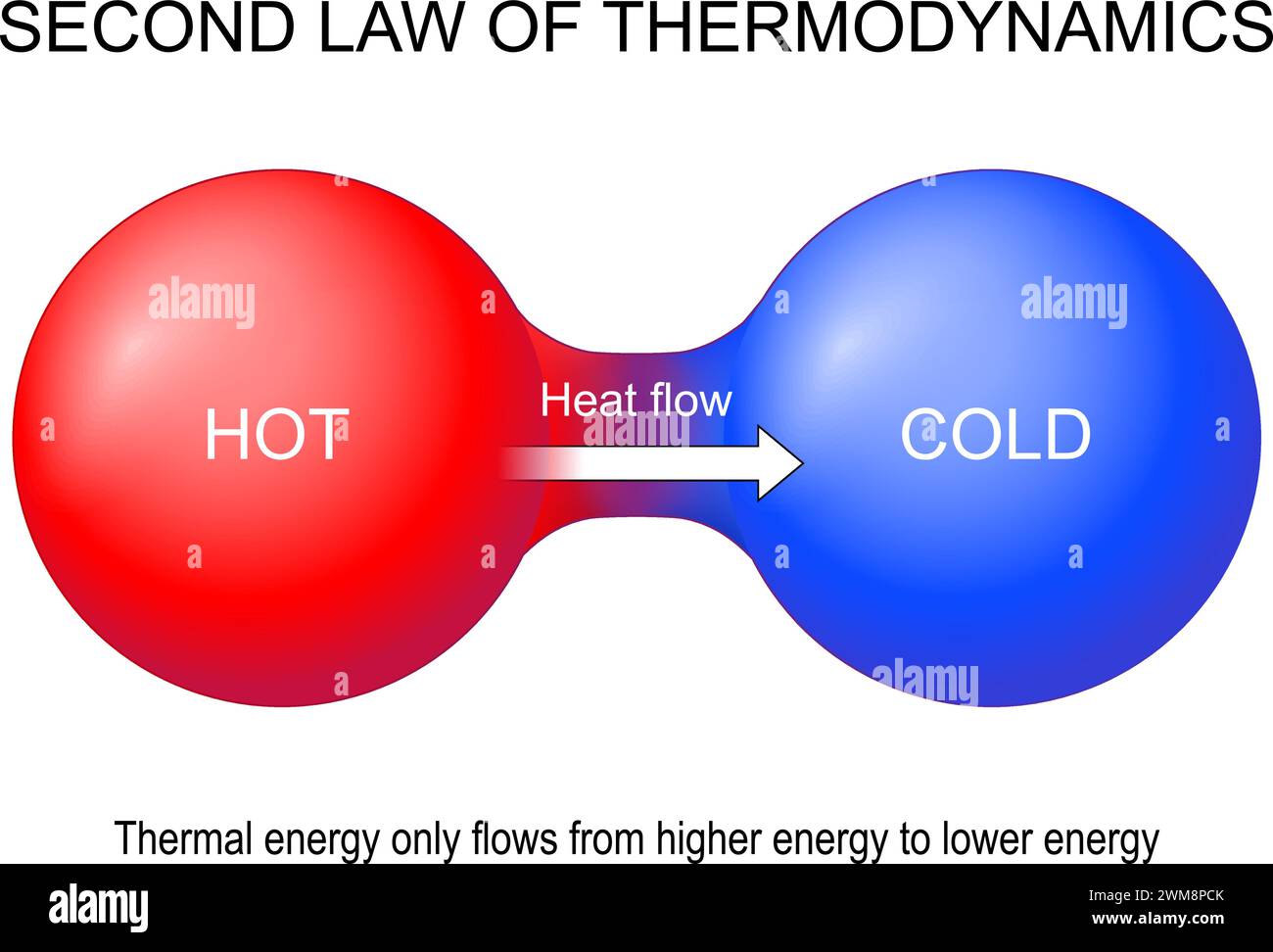 Second law of thermodynamics. Thermal energy only flows from higher energy to lower energy. Heat transfer. Entropy generation. Thermal equilibrium. Ve Stock Vectorhttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/second-law-of-thermodynamics-thermal-energy-only-flows-from-higher-energy-to-lower-energy-heat-transfer-entropy-generation-thermal-equilibrium-ve-image597638851.html
Second law of thermodynamics. Thermal energy only flows from higher energy to lower energy. Heat transfer. Entropy generation. Thermal equilibrium. Ve Stock Vectorhttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/second-law-of-thermodynamics-thermal-energy-only-flows-from-higher-energy-to-lower-energy-heat-transfer-entropy-generation-thermal-equilibrium-ve-image597638851.htmlRF2WM8PCK–Second law of thermodynamics. Thermal energy only flows from higher energy to lower energy. Heat transfer. Entropy generation. Thermal equilibrium. Ve
 Incense smoke writing 'you' or 'yes' Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/stock-image-incense-smoke-writing-you-or-yes-169817371.html
Incense smoke writing 'you' or 'yes' Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/stock-image-incense-smoke-writing-you-or-yes-169817371.htmlRFKT7RP3–Incense smoke writing 'you' or 'yes'
 Word entropy on dictionary page, macro close-up Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/word-entropy-on-dictionary-page-macro-close-up-image599133893.html
Word entropy on dictionary page, macro close-up Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/word-entropy-on-dictionary-page-macro-close-up-image599133893.htmlRM2WPMWB1–Word entropy on dictionary page, macro close-up
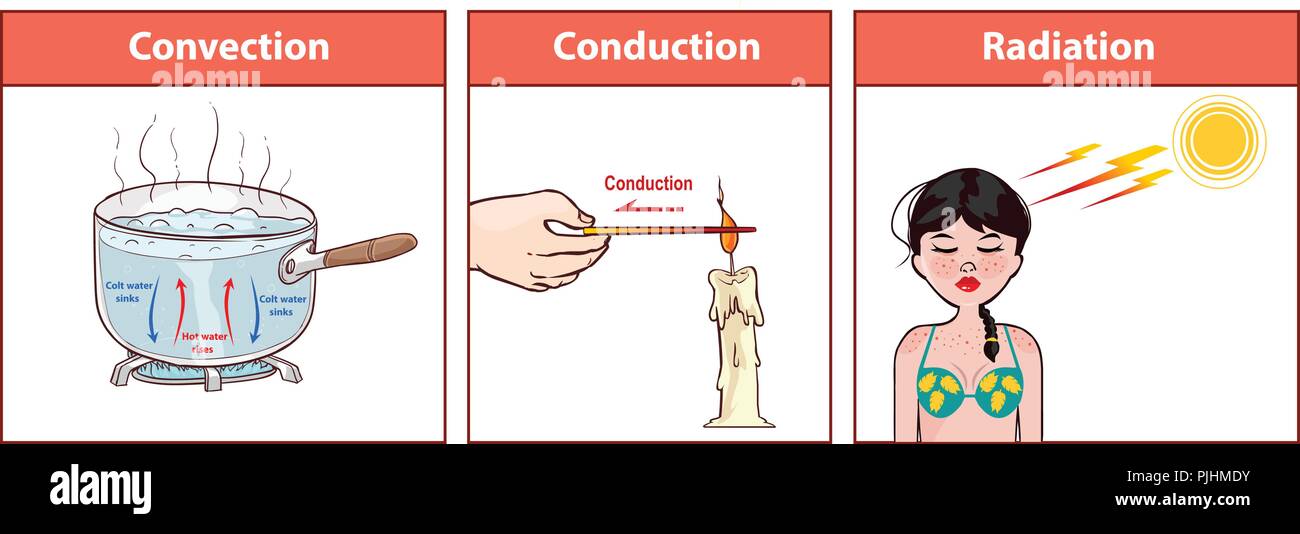 vector illustration of a Heat Transfer Stock Vectorhttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/vector-illustration-of-a-heat-transfer-image217977479.html
vector illustration of a Heat Transfer Stock Vectorhttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/vector-illustration-of-a-heat-transfer-image217977479.htmlRFPJHMDY–vector illustration of a Heat Transfer
 Bulletin of the University of Rhode Island : catalog number . COLLEGE CATALOGUE. 65 in Engineering. One and one-half laboratory credits per week, second term. Re-quired of Sophomores in Civil Engineering. Mr. Eldred. IX. Steam Engineering.—Boilers and Thermodynamics. Boilers.—Types, advantages and disadvantages, fuels, flue-gases, heat losses,corrosion and incrustation, strength, accessories, valves, piping, shop practice,design. Text, Peabody and Miller. Thermodynamics.—Mathematical discussion of laws, application to perfectgases, saturated vapors, superheated vapors, flow of fluids, engine e Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/bulletin-of-the-university-of-rhode-island-catalog-number-college-catalogue-65-in-engineering-one-and-one-half-laboratory-credits-per-week-second-term-re-quired-of-sophomores-in-civil-engineering-mr-eldred-ix-steam-engineeringboilers-and-thermodynamics-boilerstypes-advantages-and-disadvantages-fuels-flue-gases-heat-lossescorrosion-and-incrustation-strength-accessories-valves-piping-shop-practicedesign-text-peabody-and-miller-thermodynamicsmathematical-discussion-of-laws-application-to-perfectgases-saturated-vapors-superheated-vapors-flow-of-fluids-engine-e-image342867709.html
Bulletin of the University of Rhode Island : catalog number . COLLEGE CATALOGUE. 65 in Engineering. One and one-half laboratory credits per week, second term. Re-quired of Sophomores in Civil Engineering. Mr. Eldred. IX. Steam Engineering.—Boilers and Thermodynamics. Boilers.—Types, advantages and disadvantages, fuels, flue-gases, heat losses,corrosion and incrustation, strength, accessories, valves, piping, shop practice,design. Text, Peabody and Miller. Thermodynamics.—Mathematical discussion of laws, application to perfectgases, saturated vapors, superheated vapors, flow of fluids, engine e Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/bulletin-of-the-university-of-rhode-island-catalog-number-college-catalogue-65-in-engineering-one-and-one-half-laboratory-credits-per-week-second-term-re-quired-of-sophomores-in-civil-engineering-mr-eldred-ix-steam-engineeringboilers-and-thermodynamics-boilerstypes-advantages-and-disadvantages-fuels-flue-gases-heat-lossescorrosion-and-incrustation-strength-accessories-valves-piping-shop-practicedesign-text-peabody-and-miller-thermodynamicsmathematical-discussion-of-laws-application-to-perfectgases-saturated-vapors-superheated-vapors-flow-of-fluids-engine-e-image342867709.htmlRM2AWPY79–Bulletin of the University of Rhode Island : catalog number . COLLEGE CATALOGUE. 65 in Engineering. One and one-half laboratory credits per week, second term. Re-quired of Sophomores in Civil Engineering. Mr. Eldred. IX. Steam Engineering.—Boilers and Thermodynamics. Boilers.—Types, advantages and disadvantages, fuels, flue-gases, heat losses,corrosion and incrustation, strength, accessories, valves, piping, shop practice,design. Text, Peabody and Miller. Thermodynamics.—Mathematical discussion of laws, application to perfectgases, saturated vapors, superheated vapors, flow of fluids, engine e
 vector illustration of a Heat Transfer Stock Vectorhttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/vector-illustration-of-a-heat-transfer-image217977493.html
vector illustration of a Heat Transfer Stock Vectorhttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/vector-illustration-of-a-heat-transfer-image217977493.htmlRFPJHMED–vector illustration of a Heat Transfer
 North American Natural Gas Water Heater Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/stock-photo-north-american-natural-gas-water-heater-10923458.html
North American Natural Gas Water Heater Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/stock-photo-north-american-natural-gas-water-heater-10923458.htmlRFA43MXY–North American Natural Gas Water Heater
 air source heat pump mounted on a brick exterior, efficiently harnessing outdoor air to provide sustainable heating and cooling for a contemporary home. warmte pomp translation air source heat pump Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/air-source-heat-pump-mounted-on-a-brick-exterior-efficiently-harnessing-outdoor-air-to-provide-sustainable-heating-and-cooling-for-a-contemporary-home-warmte-pomp-translation-air-source-heat-pump-image615813536.html
air source heat pump mounted on a brick exterior, efficiently harnessing outdoor air to provide sustainable heating and cooling for a contemporary home. warmte pomp translation air source heat pump Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/air-source-heat-pump-mounted-on-a-brick-exterior-efficiently-harnessing-outdoor-air-to-provide-sustainable-heating-and-cooling-for-a-contemporary-home-warmte-pomp-translation-air-source-heat-pump-image615813536.htmlRF2XNTMCG–air source heat pump mounted on a brick exterior, efficiently harnessing outdoor air to provide sustainable heating and cooling for a contemporary home. warmte pomp translation air source heat pump
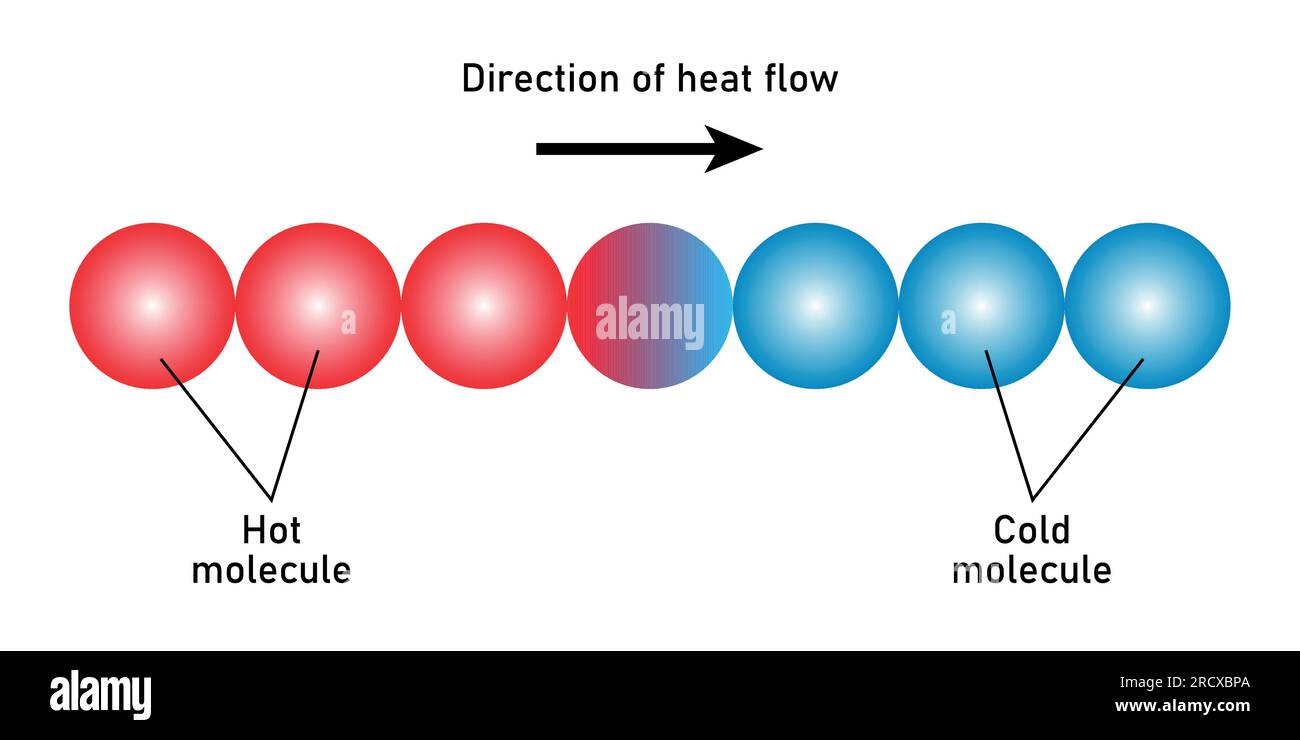 Direction of heat flow diagram. Scientific vector illustration isolated on white background. Stock Vectorhttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/direction-of-heat-flow-diagram-scientific-vector-illustration-isolated-on-white-background-image558687650.html
Direction of heat flow diagram. Scientific vector illustration isolated on white background. Stock Vectorhttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/direction-of-heat-flow-diagram-scientific-vector-illustration-isolated-on-white-background-image558687650.htmlRF2RCXBPA–Direction of heat flow diagram. Scientific vector illustration isolated on white background.
 3D illustration of 'SECOND LAW of THERMODYNAMICS' title on a tripod display board Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/3d-illustration-of-second-law-of-thermodynamics-title-on-a-tripod-image155606202.html
3D illustration of 'SECOND LAW of THERMODYNAMICS' title on a tripod display board Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/3d-illustration-of-second-law-of-thermodynamics-title-on-a-tripod-image155606202.htmlRFK14D8A–3D illustration of 'SECOND LAW of THERMODYNAMICS' title on a tripod display board
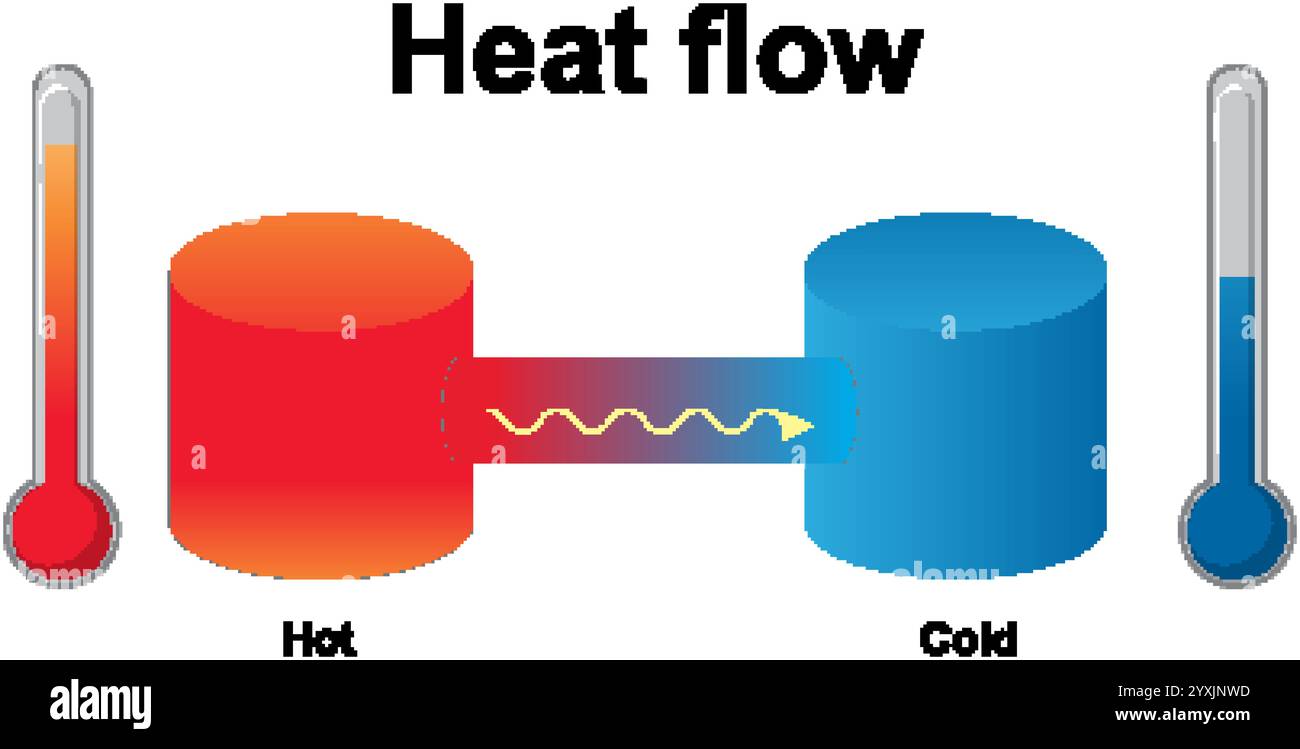 Illustration of heat flow from hot to cold Stock Vectorhttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/illustration-of-heat-flow-from-hot-to-cold-image635966617.html
Illustration of heat flow from hot to cold Stock Vectorhttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/illustration-of-heat-flow-from-hot-to-cold-image635966617.htmlRF2YXJNWD–Illustration of heat flow from hot to cold
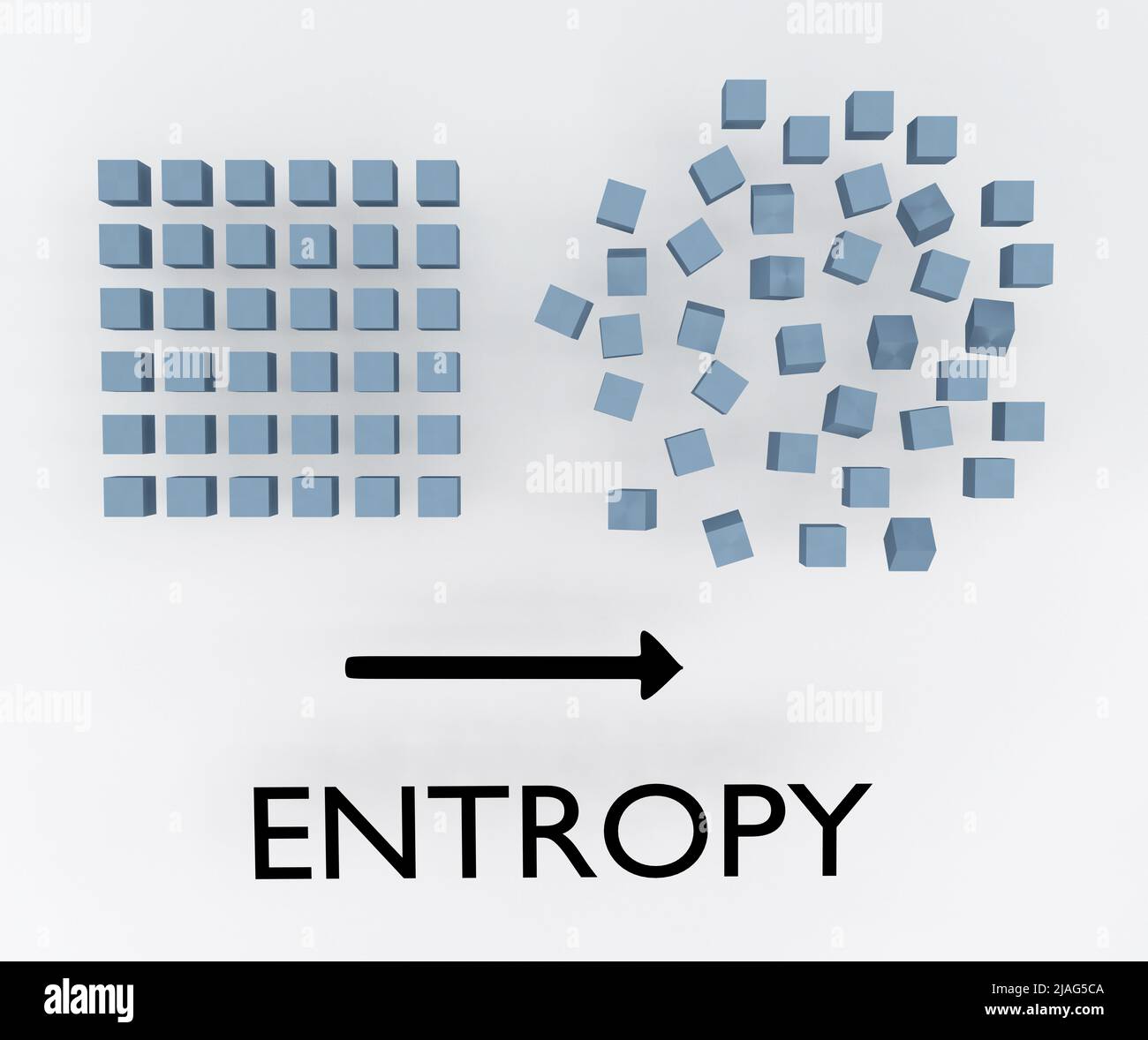 3D illustration of 36 cubes arranged in 6 rows and 6 columns, tansforming through entropy into a scattered pile, with an arrow above the script ENTROP Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/3d-illustration-of-36-cubes-arranged-in-6-rows-and-6-columns-tansforming-through-entropy-into-a-scattered-pile-with-an-arrow-above-the-script-entrop-image471181994.html
3D illustration of 36 cubes arranged in 6 rows and 6 columns, tansforming through entropy into a scattered pile, with an arrow above the script ENTROP Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/3d-illustration-of-36-cubes-arranged-in-6-rows-and-6-columns-tansforming-through-entropy-into-a-scattered-pile-with-an-arrow-above-the-script-entrop-image471181994.htmlRF2JAG5CA–3D illustration of 36 cubes arranged in 6 rows and 6 columns, tansforming through entropy into a scattered pile, with an arrow above the script ENTROP
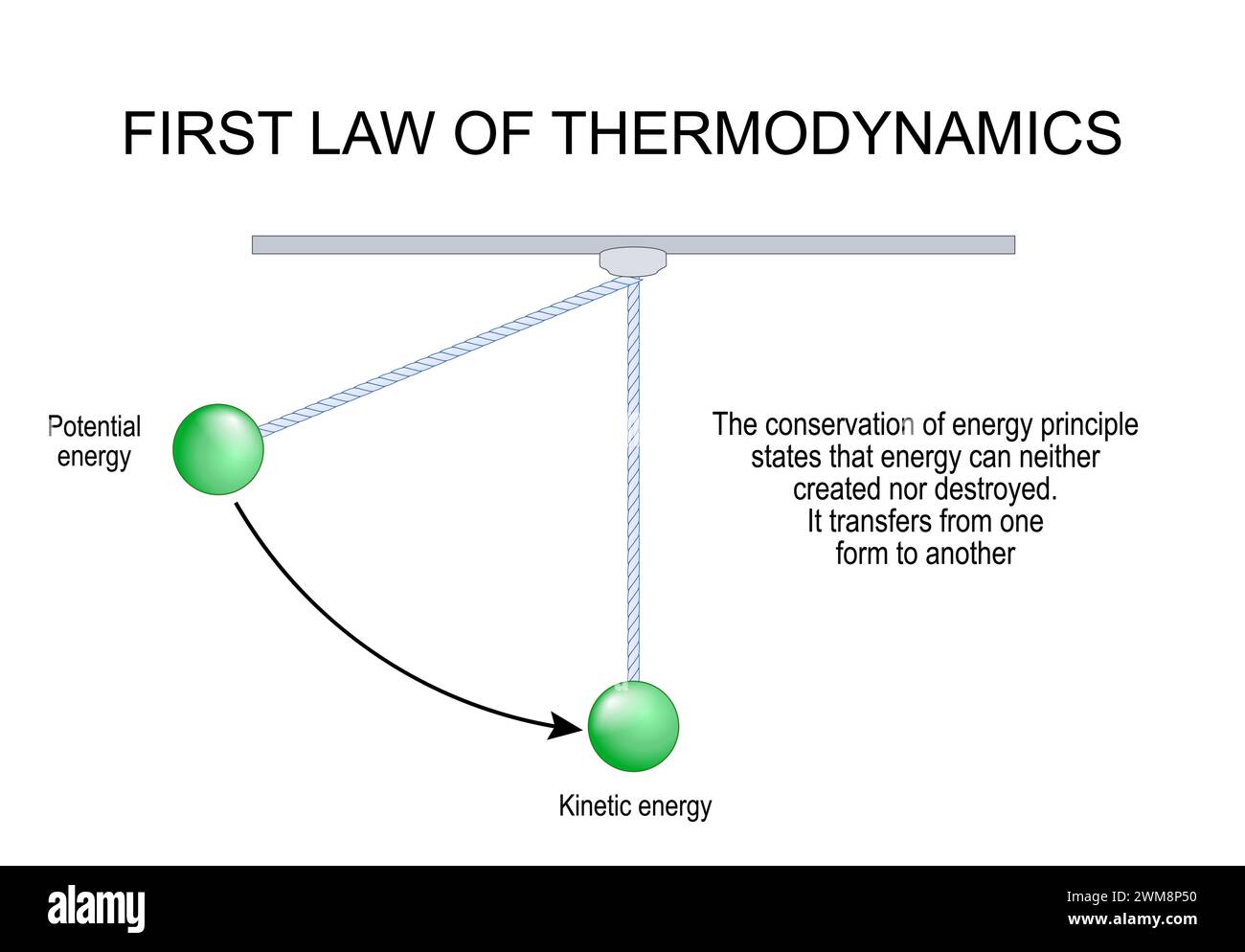 First Law of Thermodynamics. Energy transfer and Conservation. Thermodynamic equilibrium. The conservation of energy principle states that energy can Stock Vectorhttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/first-law-of-thermodynamics-energy-transfer-and-conservation-thermodynamic-equilibrium-the-conservation-of-energy-principle-states-that-energy-can-image597638636.html
First Law of Thermodynamics. Energy transfer and Conservation. Thermodynamic equilibrium. The conservation of energy principle states that energy can Stock Vectorhttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/first-law-of-thermodynamics-energy-transfer-and-conservation-thermodynamic-equilibrium-the-conservation-of-energy-principle-states-that-energy-can-image597638636.htmlRF2WM8P50–First Law of Thermodynamics. Energy transfer and Conservation. Thermodynamic equilibrium. The conservation of energy principle states that energy can
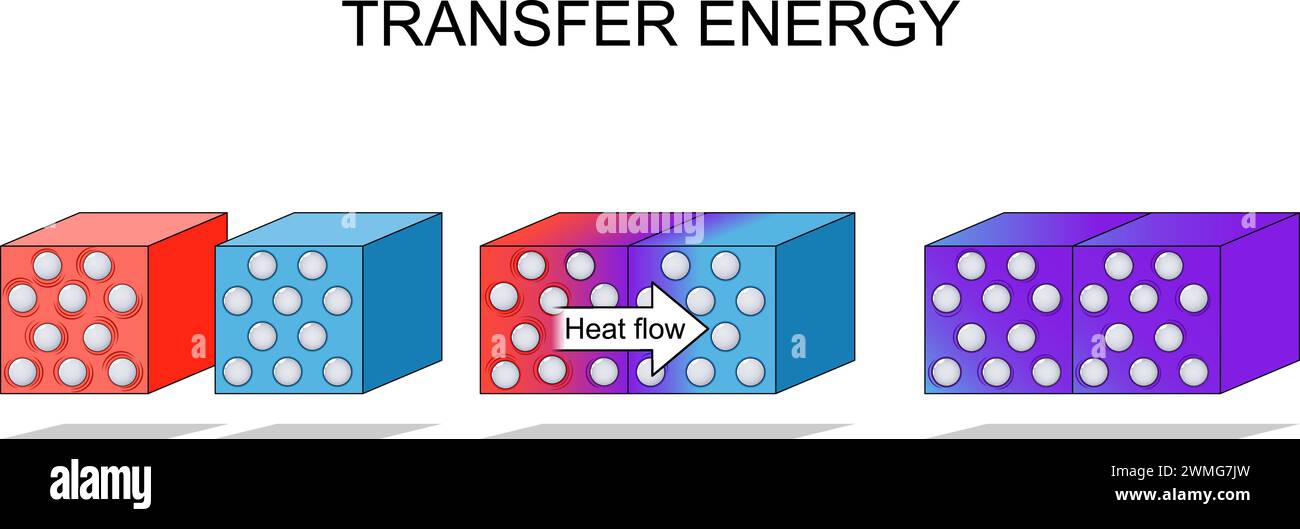 Energy transfer. law of thermodynamics. A molecular view of energy transfer between hot and cold cubes. Vector illustration Stock Vectorhttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/energy-transfer-law-of-thermodynamics-a-molecular-view-of-energy-transfer-between-hot-and-cold-cubes-vector-illustration-image597802881.html
Energy transfer. law of thermodynamics. A molecular view of energy transfer between hot and cold cubes. Vector illustration Stock Vectorhttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/energy-transfer-law-of-thermodynamics-a-molecular-view-of-energy-transfer-between-hot-and-cold-cubes-vector-illustration-image597802881.htmlRF2WMG7JW–Energy transfer. law of thermodynamics. A molecular view of energy transfer between hot and cold cubes. Vector illustration
 Word entropy on dictionary page, macro close-up Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/word-entropy-on-dictionary-page-macro-close-up-image599133885.html
Word entropy on dictionary page, macro close-up Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/word-entropy-on-dictionary-page-macro-close-up-image599133885.htmlRM2WPMWAN–Word entropy on dictionary page, macro close-up
 Third law of thermodynamics. Entropy at absolute zero. The entropy of a closed system at thermodynamic equilibrium approaches a constant value when it Stock Vectorhttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/third-law-of-thermodynamics-entropy-at-absolute-zero-the-entropy-of-a-closed-system-at-thermodynamic-equilibrium-approaches-a-constant-value-when-it-image597638947.html
Third law of thermodynamics. Entropy at absolute zero. The entropy of a closed system at thermodynamic equilibrium approaches a constant value when it Stock Vectorhttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/third-law-of-thermodynamics-entropy-at-absolute-zero-the-entropy-of-a-closed-system-at-thermodynamic-equilibrium-approaches-a-constant-value-when-it-image597638947.htmlRF2WM8PG3–Third law of thermodynamics. Entropy at absolute zero. The entropy of a closed system at thermodynamic equilibrium approaches a constant value when it
 vector illustration of a Heat Transfer Stock Vectorhttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/vector-illustration-of-a-heat-transfer-image217977474.html
vector illustration of a Heat Transfer Stock Vectorhttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/vector-illustration-of-a-heat-transfer-image217977474.htmlRFPJHMDP–vector illustration of a Heat Transfer
 Heat engineering; a text book of applied thermodynamics for engineers and students in technical schools . warm substance flowing in the oppositedirection from the flow of the cool substance the arrangement iscalled counter flow (Fig. 27). A minus sign is required beforethe dtc as the temperature decreases with an increase of dF. If tic is the temperature of the cool substance at the end cor-responding to the entrance of the warm substance and t2c is thatof the cool substance at the point of exit of the warm substance,the following is true. Mhch(tlh - t2h) = T Mccc(tlc - t^) (14) HEAT TRANSMISS Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/heat-engineering-a-text-book-of-applied-thermodynamics-for-engineers-and-students-in-technical-schools-warm-substance-flowing-in-the-oppositedirection-from-the-flow-of-the-cool-substance-the-arrangement-iscalled-counter-flow-fig-27-a-minus-sign-is-required-beforethe-dtc-as-the-temperature-decreases-with-an-increase-of-df-if-tic-is-the-temperature-of-the-cool-substance-at-the-end-cor-responding-to-the-entrance-of-the-warm-substance-and-t2c-is-thatof-the-cool-substance-at-the-point-of-exit-of-the-warm-substancethe-following-is-true-mhchtlh-t2h-=-t-mccctlc-t-14-heat-transmiss-image340285580.html
Heat engineering; a text book of applied thermodynamics for engineers and students in technical schools . warm substance flowing in the oppositedirection from the flow of the cool substance the arrangement iscalled counter flow (Fig. 27). A minus sign is required beforethe dtc as the temperature decreases with an increase of dF. If tic is the temperature of the cool substance at the end cor-responding to the entrance of the warm substance and t2c is thatof the cool substance at the point of exit of the warm substance,the following is true. Mhch(tlh - t2h) = T Mccc(tlc - t^) (14) HEAT TRANSMISS Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/heat-engineering-a-text-book-of-applied-thermodynamics-for-engineers-and-students-in-technical-schools-warm-substance-flowing-in-the-oppositedirection-from-the-flow-of-the-cool-substance-the-arrangement-iscalled-counter-flow-fig-27-a-minus-sign-is-required-beforethe-dtc-as-the-temperature-decreases-with-an-increase-of-df-if-tic-is-the-temperature-of-the-cool-substance-at-the-end-cor-responding-to-the-entrance-of-the-warm-substance-and-t2c-is-thatof-the-cool-substance-at-the-point-of-exit-of-the-warm-substancethe-following-is-true-mhchtlh-t2h-=-t-mccctlc-t-14-heat-transmiss-image340285580.htmlRM2ANH9MC–Heat engineering; a text book of applied thermodynamics for engineers and students in technical schools . warm substance flowing in the oppositedirection from the flow of the cool substance the arrangement iscalled counter flow (Fig. 27). A minus sign is required beforethe dtc as the temperature decreases with an increase of dF. If tic is the temperature of the cool substance at the end cor-responding to the entrance of the warm substance and t2c is thatof the cool substance at the point of exit of the warm substance,the following is true. Mhch(tlh - t2h) = T Mccc(tlc - t^) (14) HEAT TRANSMISS
 North American Natural Gas Water Heater Control Side Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/stock-photo-north-american-natural-gas-water-heater-control-side-10923463.html
North American Natural Gas Water Heater Control Side Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/stock-photo-north-american-natural-gas-water-heater-control-side-10923463.htmlRFA43MYM–North American Natural Gas Water Heater Control Side
 vector illustration of a Heat Transfer Stock Vectorhttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/vector-illustration-of-a-heat-transfer-image217977468.html
vector illustration of a Heat Transfer Stock Vectorhttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/vector-illustration-of-a-heat-transfer-image217977468.htmlRFPJHMDG–vector illustration of a Heat Transfer
 Heat engineering; a text book of applied thermodynamics for engineers and students in technical schools . Fig. 125.—Stodolas apparatus for exploring nozzles. and one with them inclined against the direction of flow. Thereadings of these varied, due to the impact of the steam increasingthe pressure in one and decreasing it in the other. The meanvalue was used. In addition normal holes were introduced into STEAM NOZZLES, INJECTORS, STEAM TURBINES 271 the side wall of one tube as shown. The pressures at the largeend of the nozzle showed that for this part the curve of the valueof y = 0.10 agreed Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/heat-engineering-a-text-book-of-applied-thermodynamics-for-engineers-and-students-in-technical-schools-fig-125stodolas-apparatus-for-exploring-nozzles-and-one-with-them-inclined-against-the-direction-of-flow-thereadings-of-these-varied-due-to-the-impact-of-the-steam-increasingthe-pressure-in-one-and-decreasing-it-in-the-other-the-meanvalue-was-used-in-addition-normal-holes-were-introduced-into-steam-nozzles-injectors-steam-turbines-271-the-side-wall-of-one-tube-as-shown-the-pressures-at-the-largeend-of-the-nozzle-showed-that-for-this-part-the-curve-of-the-valueof-y-=-010-agreed-image340262389.html
Heat engineering; a text book of applied thermodynamics for engineers and students in technical schools . Fig. 125.—Stodolas apparatus for exploring nozzles. and one with them inclined against the direction of flow. Thereadings of these varied, due to the impact of the steam increasingthe pressure in one and decreasing it in the other. The meanvalue was used. In addition normal holes were introduced into STEAM NOZZLES, INJECTORS, STEAM TURBINES 271 the side wall of one tube as shown. The pressures at the largeend of the nozzle showed that for this part the curve of the valueof y = 0.10 agreed Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/heat-engineering-a-text-book-of-applied-thermodynamics-for-engineers-and-students-in-technical-schools-fig-125stodolas-apparatus-for-exploring-nozzles-and-one-with-them-inclined-against-the-direction-of-flow-thereadings-of-these-varied-due-to-the-impact-of-the-steam-increasingthe-pressure-in-one-and-decreasing-it-in-the-other-the-meanvalue-was-used-in-addition-normal-holes-were-introduced-into-steam-nozzles-injectors-steam-turbines-271-the-side-wall-of-one-tube-as-shown-the-pressures-at-the-largeend-of-the-nozzle-showed-that-for-this-part-the-curve-of-the-valueof-y-=-010-agreed-image340262389.htmlRM2ANG845–Heat engineering; a text book of applied thermodynamics for engineers and students in technical schools . Fig. 125.—Stodolas apparatus for exploring nozzles. and one with them inclined against the direction of flow. Thereadings of these varied, due to the impact of the steam increasingthe pressure in one and decreasing it in the other. The meanvalue was used. In addition normal holes were introduced into STEAM NOZZLES, INJECTORS, STEAM TURBINES 271 the side wall of one tube as shown. The pressures at the largeend of the nozzle showed that for this part the curve of the valueof y = 0.10 agreed
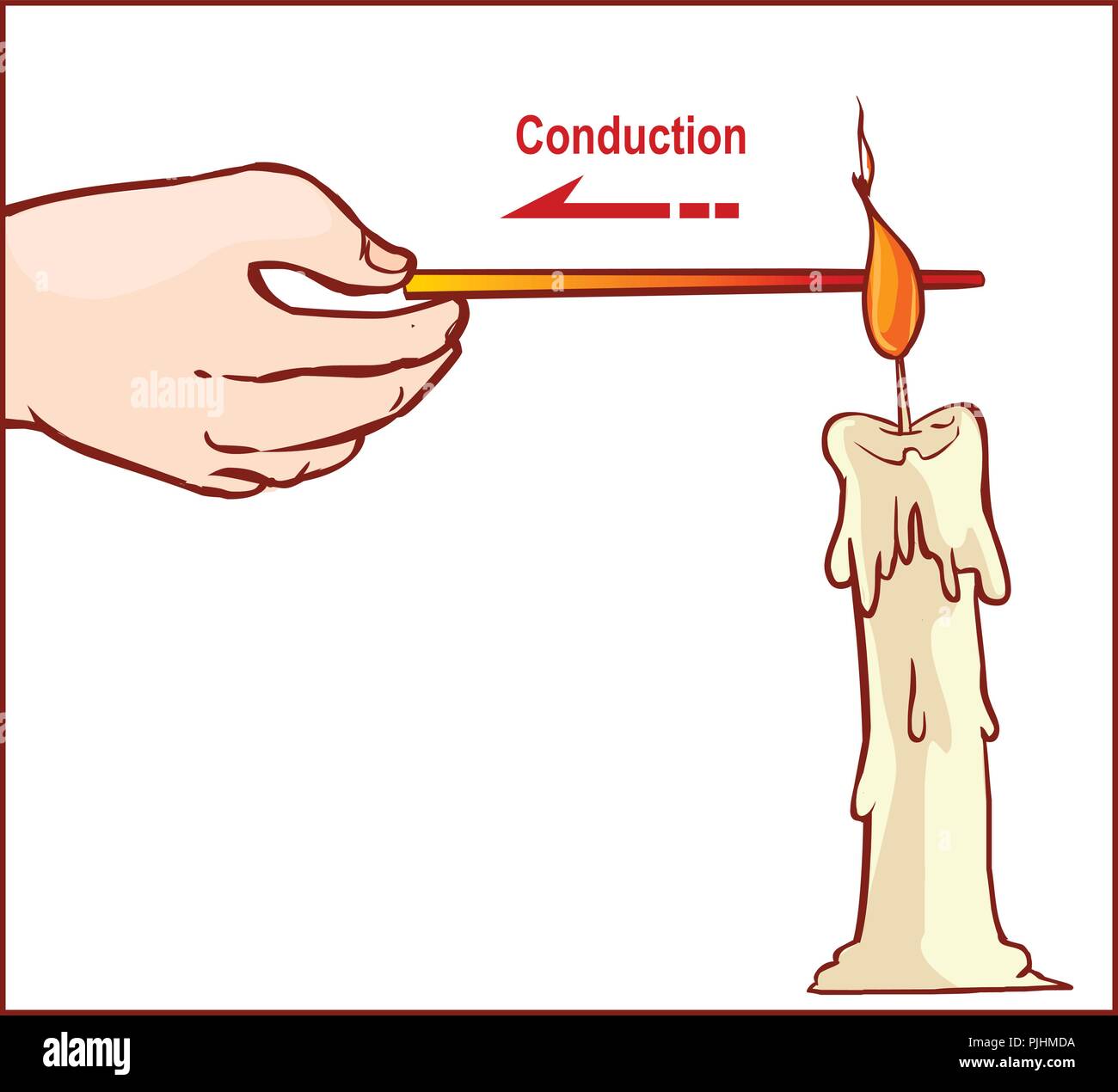 vector illustration of a Heat Transfer Stock Vectorhttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/vector-illustration-of-a-heat-transfer-image217977462.html
vector illustration of a Heat Transfer Stock Vectorhttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/vector-illustration-of-a-heat-transfer-image217977462.htmlRFPJHMDA–vector illustration of a Heat Transfer
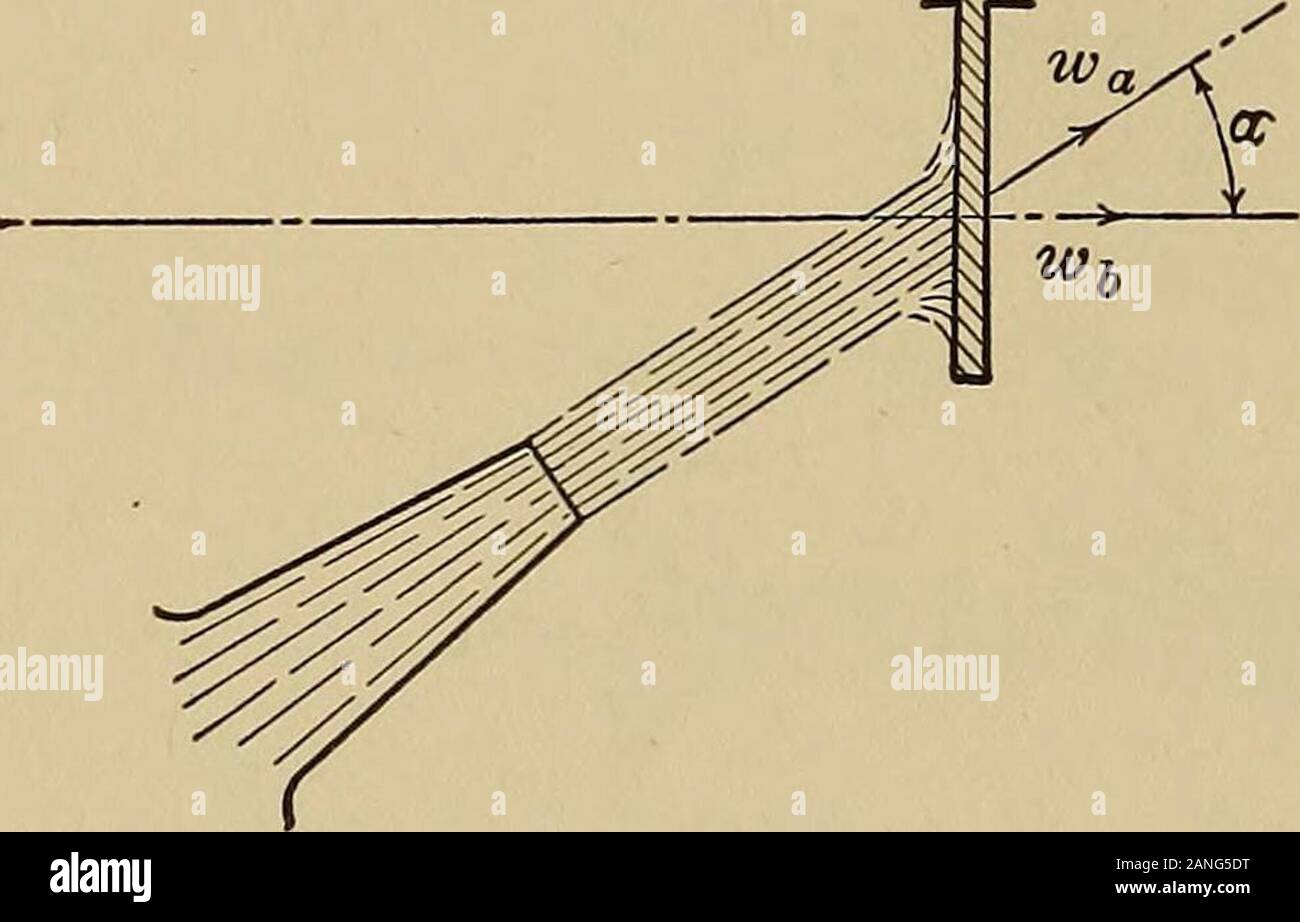 Heat engineering; a text book of applied thermodynamics for engineers and students in technical schools . he expression must be divided by g giving m(wa — wp) P =If wb is made zero this becomes P = mwa (26) (27) In this latter case there is no work done as the blade is sta- 288 HEAT ENGINEERING tionary. The force is called the force of impact or the impulse ofthe jet. The nozzle of the j et must feel a force equal to this as someforce must be required to produce this flow. This is called thereaction of the jet. The word impulse refers to the force exertedby the jet when it strikes a body while Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/heat-engineering-a-text-book-of-applied-thermodynamics-for-engineers-and-students-in-technical-schools-he-expression-must-be-divided-by-g-giving-mwa-wp-p-=if-wb-is-made-zero-this-becomes-p-=-mwa-26-27-in-this-latter-case-there-is-no-work-done-as-the-blade-is-sta-288-heat-engineering-tionary-the-force-is-called-the-force-of-impact-or-the-impulse-ofthe-jet-the-nozzle-of-the-j-et-must-feel-a-force-equal-to-this-as-someforce-must-be-required-to-produce-this-flow-this-is-called-thereaction-of-the-jet-the-word-impulse-refers-to-the-force-exertedby-the-jet-when-it-strikes-a-body-while-image340260308.html
Heat engineering; a text book of applied thermodynamics for engineers and students in technical schools . he expression must be divided by g giving m(wa — wp) P =If wb is made zero this becomes P = mwa (26) (27) In this latter case there is no work done as the blade is sta- 288 HEAT ENGINEERING tionary. The force is called the force of impact or the impulse ofthe jet. The nozzle of the j et must feel a force equal to this as someforce must be required to produce this flow. This is called thereaction of the jet. The word impulse refers to the force exertedby the jet when it strikes a body while Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/heat-engineering-a-text-book-of-applied-thermodynamics-for-engineers-and-students-in-technical-schools-he-expression-must-be-divided-by-g-giving-mwa-wp-p-=if-wb-is-made-zero-this-becomes-p-=-mwa-26-27-in-this-latter-case-there-is-no-work-done-as-the-blade-is-sta-288-heat-engineering-tionary-the-force-is-called-the-force-of-impact-or-the-impulse-ofthe-jet-the-nozzle-of-the-j-et-must-feel-a-force-equal-to-this-as-someforce-must-be-required-to-produce-this-flow-this-is-called-thereaction-of-the-jet-the-word-impulse-refers-to-the-force-exertedby-the-jet-when-it-strikes-a-body-while-image340260308.htmlRM2ANG5DT–Heat engineering; a text book of applied thermodynamics for engineers and students in technical schools . he expression must be divided by g giving m(wa — wp) P =If wb is made zero this becomes P = mwa (26) (27) In this latter case there is no work done as the blade is sta- 288 HEAT ENGINEERING tionary. The force is called the force of impact or the impulse ofthe jet. The nozzle of the j et must feel a force equal to this as someforce must be required to produce this flow. This is called thereaction of the jet. The word impulse refers to the force exertedby the jet when it strikes a body while
 vector illustration of a Heat Transfer Stock Vectorhttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/vector-illustration-of-a-heat-transfer-image217977473.html
vector illustration of a Heat Transfer Stock Vectorhttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/vector-illustration-of-a-heat-transfer-image217977473.htmlRFPJHMDN–vector illustration of a Heat Transfer
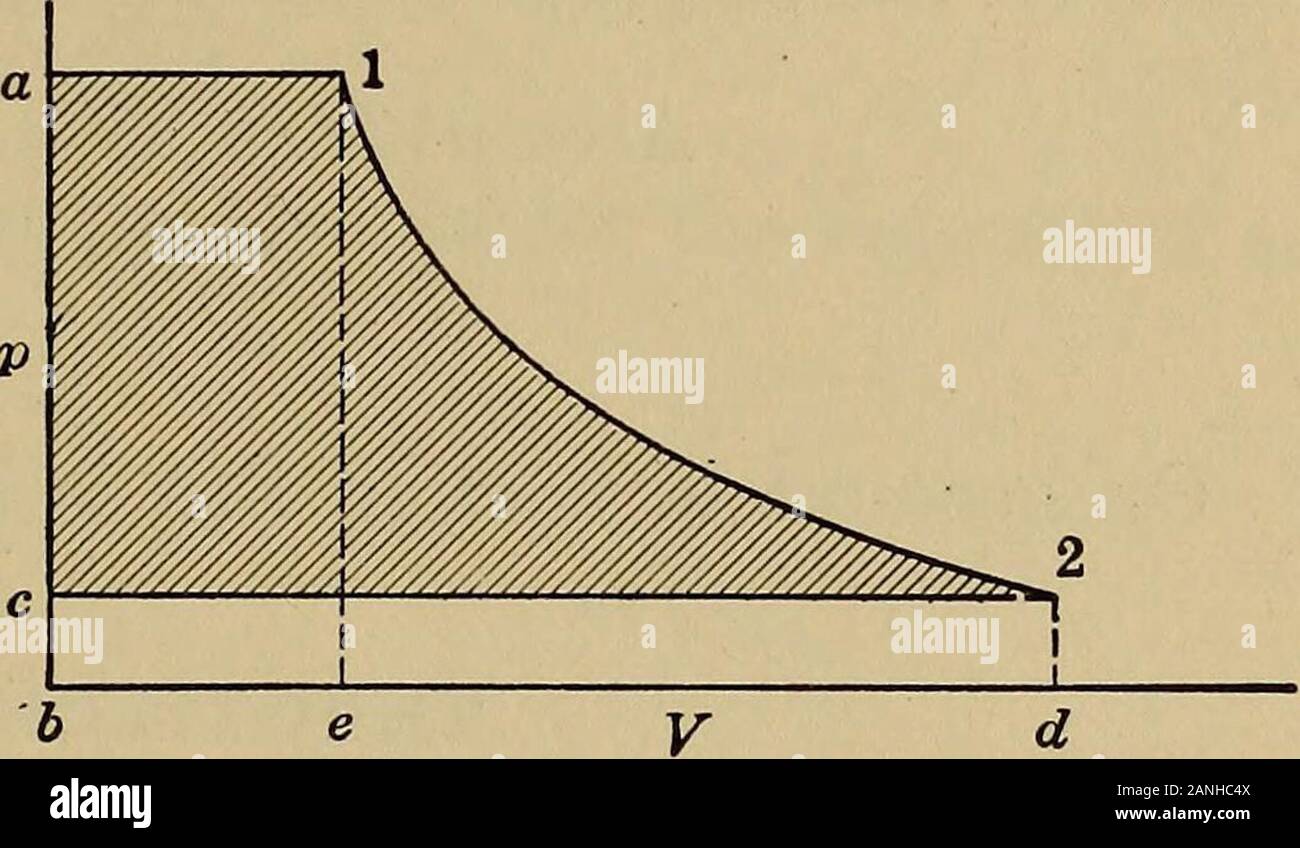 Heat engineering; a text book of applied thermodynamics for engineers and students in technical schools . Fig. 18. -TS d of flu. agram for flowds. Fig. 19.—pV diagram for flow offluids. stead of using % — i2 for these points, only a fraction of this isused. In Fig. 18 abcde is equal to i, since abcf = q cdef = xrabcde = q + xr = i — Ayv = iabg2e = i2i — i2 = cd2g This assumes no friction, hence d and 2 have the same entropy.By experiment the heat used in friction is found to be y(ii — i2),hence this must be subtracted to get the amount of heat left togive the gain in kinetic energy. Hence w Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/heat-engineering-a-text-book-of-applied-thermodynamics-for-engineers-and-students-in-technical-schools-fig-18-ts-d-of-flu-agram-for-flowds-fig-19pv-diagram-for-flow-offluids-stead-of-using-i2-for-these-points-only-a-fraction-of-this-isused-in-fig-18-abcde-is-equal-to-i-since-abcf-=-q-cdef-=-xrabcde-=-q-xr-=-i-ayv-=-iabg2e-=-i2i-i2-=-cd2g-this-assumes-no-friction-hence-d-and-2-have-the-same-entropyby-experiment-the-heat-used-in-friction-is-found-to-be-yii-i2hence-this-must-be-subtracted-to-get-the-amount-of-heat-left-togive-the-gain-in-kinetic-energy-hence-w-image340287498.html
Heat engineering; a text book of applied thermodynamics for engineers and students in technical schools . Fig. 18. -TS d of flu. agram for flowds. Fig. 19.—pV diagram for flow offluids. stead of using % — i2 for these points, only a fraction of this isused. In Fig. 18 abcde is equal to i, since abcf = q cdef = xrabcde = q + xr = i — Ayv = iabg2e = i2i — i2 = cd2g This assumes no friction, hence d and 2 have the same entropy.By experiment the heat used in friction is found to be y(ii — i2),hence this must be subtracted to get the amount of heat left togive the gain in kinetic energy. Hence w Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/heat-engineering-a-text-book-of-applied-thermodynamics-for-engineers-and-students-in-technical-schools-fig-18-ts-d-of-flu-agram-for-flowds-fig-19pv-diagram-for-flow-offluids-stead-of-using-i2-for-these-points-only-a-fraction-of-this-isused-in-fig-18-abcde-is-equal-to-i-since-abcf-=-q-cdef-=-xrabcde-=-q-xr-=-i-ayv-=-iabg2e-=-i2i-i2-=-cd2g-this-assumes-no-friction-hence-d-and-2-have-the-same-entropyby-experiment-the-heat-used-in-friction-is-found-to-be-yii-i2hence-this-must-be-subtracted-to-get-the-amount-of-heat-left-togive-the-gain-in-kinetic-energy-hence-w-image340287498.htmlRM2ANHC4X–Heat engineering; a text book of applied thermodynamics for engineers and students in technical schools . Fig. 18. -TS d of flu. agram for flowds. Fig. 19.—pV diagram for flow offluids. stead of using % — i2 for these points, only a fraction of this isused. In Fig. 18 abcde is equal to i, since abcf = q cdef = xrabcde = q + xr = i — Ayv = iabg2e = i2i — i2 = cd2g This assumes no friction, hence d and 2 have the same entropy.By experiment the heat used in friction is found to be y(ii — i2),hence this must be subtracted to get the amount of heat left togive the gain in kinetic energy. Hence w
 . Applied thermodynamics for engineers. FiG. 305. Art. 626. — Vapor Cycle. VAPOR REFRIGERATION 463. 464 APPLIED THERMODYNAMICS sure, and the absorption of heat from surrounding bodies duringevaporation. The pump analogy is useful. The vaporizer may belikened to a pit or well in which a fixed water level is to be main-tained ; by using a pump, the water may be raised to a level at whichit will of itself flow away. The pump is the compressor, whichraises the low-temperature heat of the vaporizer to a high-tempera-ture heat which can flow away with the condensed water. Theheat absorbed by the wat Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/applied-thermodynamics-for-engineers-fig-305-art-626-vapor-cycle-vapor-refrigeration-463-464-applied-thermodynamics-sure-and-the-absorption-of-heat-from-surrounding-bodies-duringevaporation-the-pump-analogy-is-useful-the-vaporizer-may-belikened-to-a-pit-or-well-in-which-a-fixed-water-level-is-to-be-main-tained-by-using-a-pump-the-water-may-be-raised-to-a-level-at-whichit-will-of-itself-flow-away-the-pump-is-the-compressor-whichraises-the-low-temperature-heat-of-the-vaporizer-to-a-high-tempera-ture-heat-which-can-flow-away-with-the-condensed-water-theheat-absorbed-by-the-wat-image370452613.html
. Applied thermodynamics for engineers. FiG. 305. Art. 626. — Vapor Cycle. VAPOR REFRIGERATION 463. 464 APPLIED THERMODYNAMICS sure, and the absorption of heat from surrounding bodies duringevaporation. The pump analogy is useful. The vaporizer may belikened to a pit or well in which a fixed water level is to be main-tained ; by using a pump, the water may be raised to a level at whichit will of itself flow away. The pump is the compressor, whichraises the low-temperature heat of the vaporizer to a high-tempera-ture heat which can flow away with the condensed water. Theheat absorbed by the wat Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/applied-thermodynamics-for-engineers-fig-305-art-626-vapor-cycle-vapor-refrigeration-463-464-applied-thermodynamics-sure-and-the-absorption-of-heat-from-surrounding-bodies-duringevaporation-the-pump-analogy-is-useful-the-vaporizer-may-belikened-to-a-pit-or-well-in-which-a-fixed-water-level-is-to-be-main-tained-by-using-a-pump-the-water-may-be-raised-to-a-level-at-whichit-will-of-itself-flow-away-the-pump-is-the-compressor-whichraises-the-low-temperature-heat-of-the-vaporizer-to-a-high-tempera-ture-heat-which-can-flow-away-with-the-condensed-water-theheat-absorbed-by-the-wat-image370452613.htmlRM2CEKG2D–. Applied thermodynamics for engineers. FiG. 305. Art. 626. — Vapor Cycle. VAPOR REFRIGERATION 463. 464 APPLIED THERMODYNAMICS sure, and the absorption of heat from surrounding bodies duringevaporation. The pump analogy is useful. The vaporizer may belikened to a pit or well in which a fixed water level is to be main-tained ; by using a pump, the water may be raised to a level at whichit will of itself flow away. The pump is the compressor, whichraises the low-temperature heat of the vaporizer to a high-tempera-ture heat which can flow away with the condensed water. Theheat absorbed by the wat