Quick filters:
Verbenol Stock Photos and Images
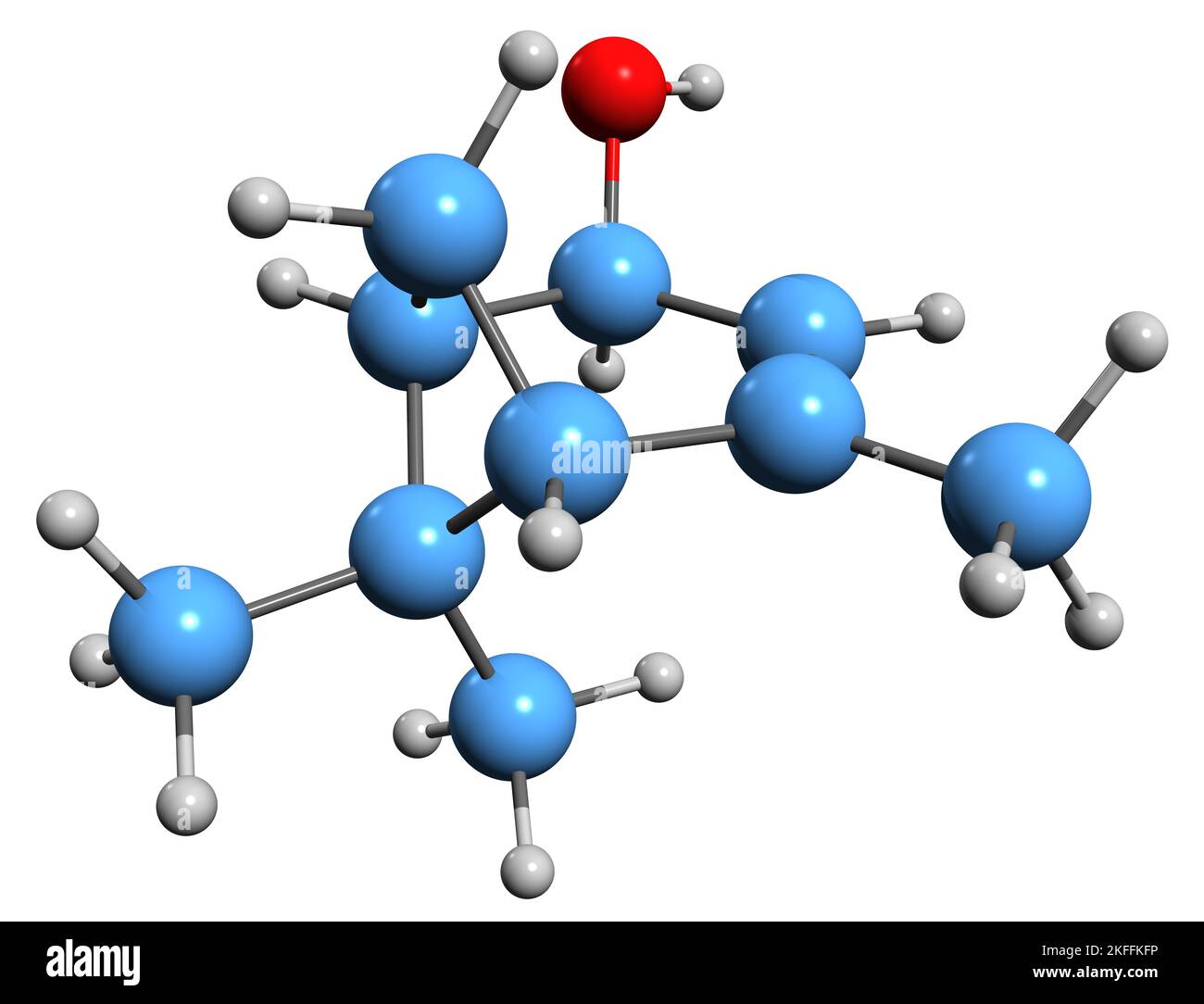 3D image of Verbenol skeletal formula - molecular chemical structure of 2-Pine-4-ol isolated on white background Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/3d-image-of-verbenol-skeletal-formula-molecular-chemical-structure-of-2-pine-4-ol-isolated-on-white-background-image491454762.html
3D image of Verbenol skeletal formula - molecular chemical structure of 2-Pine-4-ol isolated on white background Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/3d-image-of-verbenol-skeletal-formula-molecular-chemical-structure-of-2-pine-4-ol-isolated-on-white-background-image491454762.htmlRF2KFFKFP–3D image of Verbenol skeletal formula - molecular chemical structure of 2-Pine-4-ol isolated on white background
 . Annual report on essential oils, synthetic perfumes, &c. Essences and essential oils; Perfumes. C-CH3 (I) Verbenol. C-OH. CH,. Please note that these images are extracted from scanned page images that may have been digitally enhanced for readability - coloration and appearance of these illustrations may not perfectly resemble the original work.. Schimmel & Co. Miltitz bei Leipzig : Schimmel & Co. Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/annual-report-on-essential-oils-synthetic-perfumes-ampc-essences-and-essential-oils-perfumes-c-ch3-i-verbenol-c-oh-ch-please-note-that-these-images-are-extracted-from-scanned-page-images-that-may-have-been-digitally-enhanced-for-readability-coloration-and-appearance-of-these-illustrations-may-not-perfectly-resemble-the-original-work-schimmel-amp-co-miltitz-bei-leipzig-schimmel-amp-co-image236192909.html
. Annual report on essential oils, synthetic perfumes, &c. Essences and essential oils; Perfumes. C-CH3 (I) Verbenol. C-OH. CH,. Please note that these images are extracted from scanned page images that may have been digitally enhanced for readability - coloration and appearance of these illustrations may not perfectly resemble the original work.. Schimmel & Co. Miltitz bei Leipzig : Schimmel & Co. Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/annual-report-on-essential-oils-synthetic-perfumes-ampc-essences-and-essential-oils-perfumes-c-ch3-i-verbenol-c-oh-ch-please-note-that-these-images-are-extracted-from-scanned-page-images-that-may-have-been-digitally-enhanced-for-readability-coloration-and-appearance-of-these-illustrations-may-not-perfectly-resemble-the-original-work-schimmel-amp-co-miltitz-bei-leipzig-schimmel-amp-co-image236192909.htmlRMRM7ED1–. Annual report on essential oils, synthetic perfumes, &c. Essences and essential oils; Perfumes. C-CH3 (I) Verbenol. C-OH. CH,. Please note that these images are extracted from scanned page images that may have been digitally enhanced for readability - coloration and appearance of these illustrations may not perfectly resemble the original work.. Schimmel & Co. Miltitz bei Leipzig : Schimmel & Co.
 . Annual report on essential oils, synthetic perfumes, &c. Essences and essential oils; Perfumes. Notes on scientific research. double bonds was proved by the formation of the terpene from verbenol (I), by the value found for the molecular refraction (44.57, calc. for j 43.05), and by the behaviour of verbenene towards bromine. The easily-obtainable dibromide was strongly optically active, the rotation being opposite to that of the primary material, and gave rise, on treatment with dilute potash solution, to an unsaturated glycol Ci0H16O2 (IV; m. p. 141°) and to an apparently oxide-like b Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/annual-report-on-essential-oils-synthetic-perfumes-ampc-essences-and-essential-oils-perfumes-notes-on-scientific-research-double-bonds-was-proved-by-the-formation-of-the-terpene-from-verbenol-i-by-the-value-found-for-the-molecular-refraction-4457-calc-for-j-4305-and-by-the-behaviour-of-verbenene-towards-bromine-the-easily-obtainable-dibromide-was-strongly-optically-active-the-rotation-being-opposite-to-that-of-the-primary-material-and-gave-rise-on-treatment-with-dilute-potash-solution-to-an-unsaturated-glycol-ci0h16o2-iv-m-p-141-and-to-an-apparently-oxide-like-b-image236192919.html
. Annual report on essential oils, synthetic perfumes, &c. Essences and essential oils; Perfumes. Notes on scientific research. double bonds was proved by the formation of the terpene from verbenol (I), by the value found for the molecular refraction (44.57, calc. for j 43.05), and by the behaviour of verbenene towards bromine. The easily-obtainable dibromide was strongly optically active, the rotation being opposite to that of the primary material, and gave rise, on treatment with dilute potash solution, to an unsaturated glycol Ci0H16O2 (IV; m. p. 141°) and to an apparently oxide-like b Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/annual-report-on-essential-oils-synthetic-perfumes-ampc-essences-and-essential-oils-perfumes-notes-on-scientific-research-double-bonds-was-proved-by-the-formation-of-the-terpene-from-verbenol-i-by-the-value-found-for-the-molecular-refraction-4457-calc-for-j-4305-and-by-the-behaviour-of-verbenene-towards-bromine-the-easily-obtainable-dibromide-was-strongly-optically-active-the-rotation-being-opposite-to-that-of-the-primary-material-and-gave-rise-on-treatment-with-dilute-potash-solution-to-an-unsaturated-glycol-ci0h16o2-iv-m-p-141-and-to-an-apparently-oxide-like-b-image236192919.htmlRMRM7EDB–. Annual report on essential oils, synthetic perfumes, &c. Essences and essential oils; Perfumes. Notes on scientific research. double bonds was proved by the formation of the terpene from verbenol (I), by the value found for the molecular refraction (44.57, calc. for j 43.05), and by the behaviour of verbenene towards bromine. The easily-obtainable dibromide was strongly optically active, the rotation being opposite to that of the primary material, and gave rise, on treatment with dilute potash solution, to an unsaturated glycol Ci0H16O2 (IV; m. p. 141°) and to an apparently oxide-like b