Quick filters:
Yttrium oxide Stock Photos and Images
 Rare earths ceroxide, yttrium oxide and neodymium oxide, photographed at the Tradium GmbH in Frankfurt, Germany, Nov.5, 2012. Tradium is considered one of Europe's biggest traders in technology metals and rare earths. Photo: Frank Rumpenhorst/dpa Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/stock-photo-rare-earths-ceroxide-yttrium-oxide-and-neodymium-oxide-photographed-77085857.html
Rare earths ceroxide, yttrium oxide and neodymium oxide, photographed at the Tradium GmbH in Frankfurt, Germany, Nov.5, 2012. Tradium is considered one of Europe's biggest traders in technology metals and rare earths. Photo: Frank Rumpenhorst/dpa Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/stock-photo-rare-earths-ceroxide-yttrium-oxide-and-neodymium-oxide-photographed-77085857.htmlRMEDBFP9–Rare earths ceroxide, yttrium oxide and neodymium oxide, photographed at the Tradium GmbH in Frankfurt, Germany, Nov.5, 2012. Tradium is considered one of Europe's biggest traders in technology metals and rare earths. Photo: Frank Rumpenhorst/dpa
 Yttrium Oxide Crystals Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/stock-photo-yttrium-oxide-crystals-135020029.html
Yttrium Oxide Crystals Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/stock-photo-yttrium-oxide-crystals-135020029.htmlRMHRJKBW–Yttrium Oxide Crystals
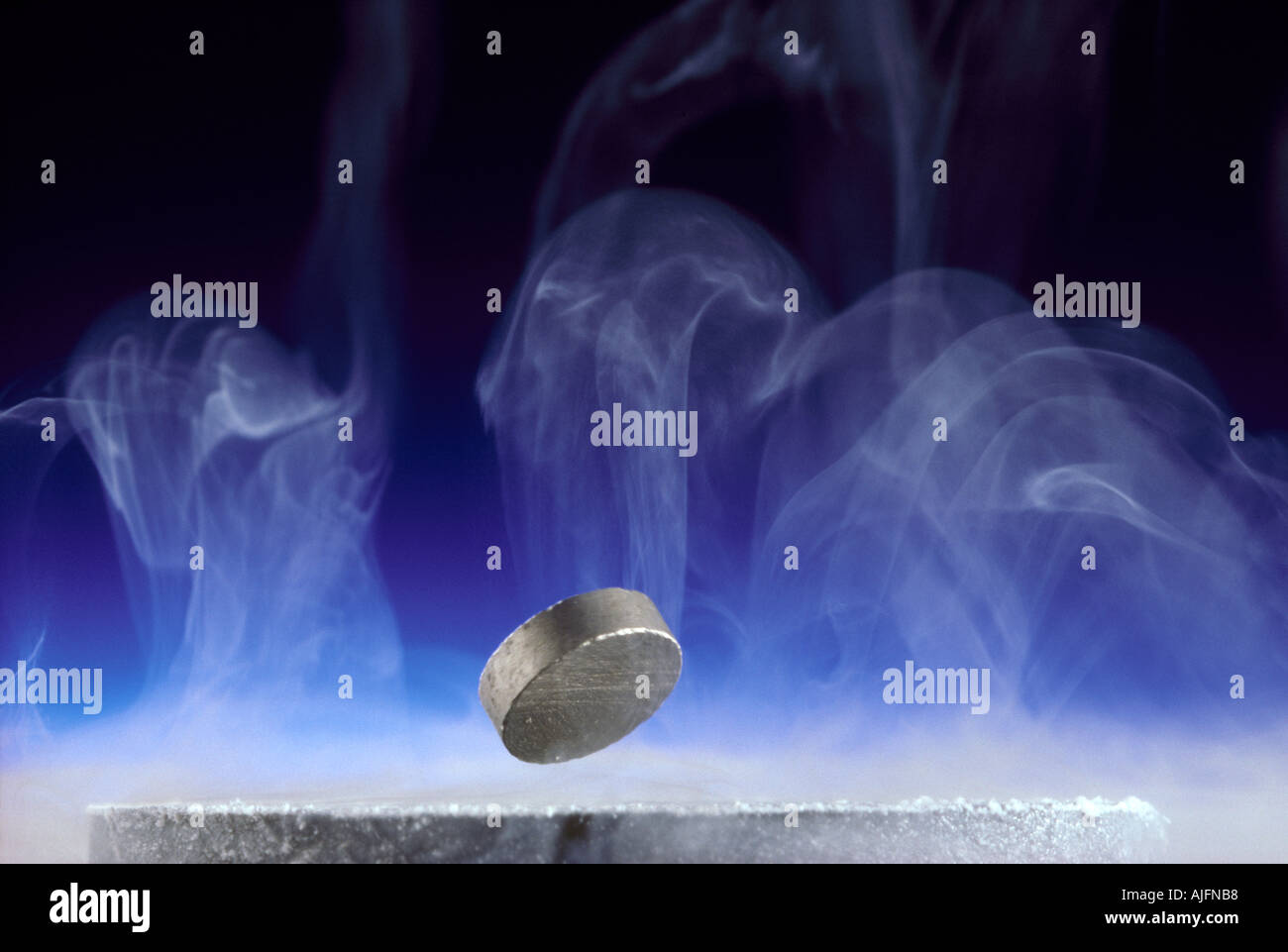 Superconducting Yttrium Barium Copper Oxide Composite Levitating Samarium Cobalt Magnet & Illustrating the Meissner Effect Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/superconducting-yttrium-barium-copper-oxide-composite-levitating-samarium-image4807095.html
Superconducting Yttrium Barium Copper Oxide Composite Levitating Samarium Cobalt Magnet & Illustrating the Meissner Effect Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/superconducting-yttrium-barium-copper-oxide-composite-levitating-samarium-image4807095.htmlRMAJFNB8–Superconducting Yttrium Barium Copper Oxide Composite Levitating Samarium Cobalt Magnet & Illustrating the Meissner Effect
 Y2O3 - Yttrium oxide. Chemical compound. CAS number 1314-36-9 Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/y2o3-yttrium-oxide-chemical-compound-cas-number-1314-36-9-image595377368.html
Y2O3 - Yttrium oxide. Chemical compound. CAS number 1314-36-9 Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/y2o3-yttrium-oxide-chemical-compound-cas-number-1314-36-9-image595377368.htmlRF2WGHNWC–Y2O3 - Yttrium oxide. Chemical compound. CAS number 1314-36-9
 Yttrium is a chemical element with the symbol Y and atomic number 39. Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/yttrium-is-a-chemical-element-with-the-symbol-y-and-atomic-number-39-image415993647.html
Yttrium is a chemical element with the symbol Y and atomic number 39. Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/yttrium-is-a-chemical-element-with-the-symbol-y-and-atomic-number-39-image415993647.htmlRM2F4P43Y–Yttrium is a chemical element with the symbol Y and atomic number 39.
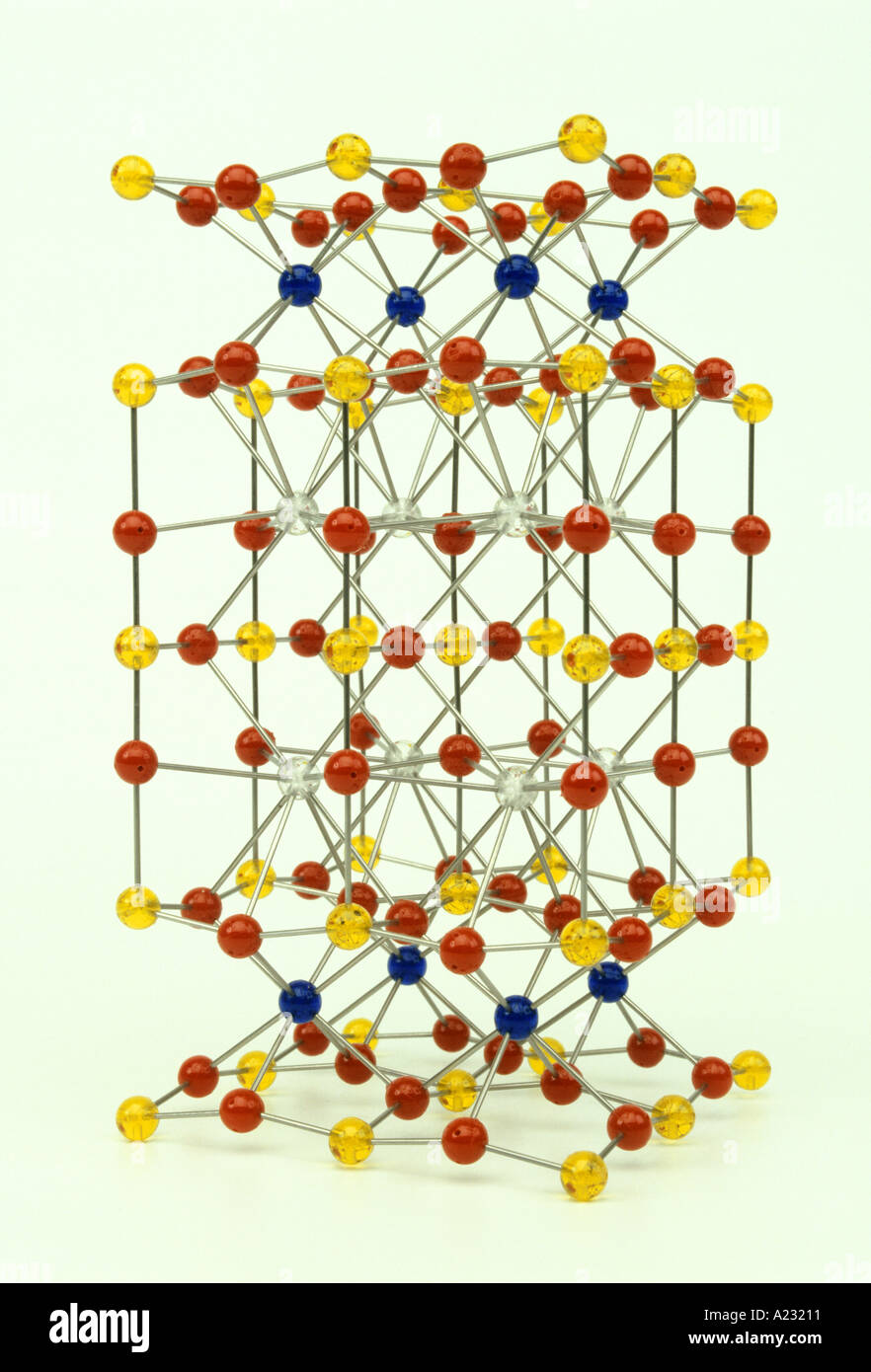 molecular model of a superconductor yttrium barium copper oxide YBa2Cu3O7 Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/molecular-model-of-a-superconductor-yttrium-barium-copper-oxide-yba2cu3o7-image143889.html
molecular model of a superconductor yttrium barium copper oxide YBa2Cu3O7 Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/molecular-model-of-a-superconductor-yttrium-barium-copper-oxide-yba2cu3o7-image143889.htmlRMA23211–molecular model of a superconductor yttrium barium copper oxide YBa2Cu3O7
 Observations on the rare earths : yttrium chloride and the atomic weight of yttrium . d tofractionation of earth mixtures containing didymium or gadolinium unless theyare first removed by the potassium sulphate method. The determination of the ratio Y2O3 : Y2(S04.)3 as a means of finding theatomic weight of yttrium was studied and found to be rather unoertain. Themethod aprarently gives atomio weight values which are lower than those gain-ed by other more reliable methods. The ratio Ya03 : 2YC13 waR studied and some very consistent results wereobtained. Weighed quantities of yttrium oxide were Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/observations-on-the-rare-earths-yttrium-chloride-and-the-atomic-weight-of-yttrium-d-tofractionation-of-earth-mixtures-containing-didymium-or-gadolinium-unless-theyare-first-removed-by-the-potassium-sulphate-method-the-determination-of-the-ratio-y2o3-y2s043-as-a-means-of-finding-theatomic-weight-of-yttrium-was-studied-and-found-to-be-rather-unoertain-themethod-aprarently-gives-atomio-weight-values-which-are-lower-than-those-gain-ed-by-other-more-reliable-methods-the-ratio-ya03-2yc13-war-studied-and-some-very-consistent-results-wereobtained-weighed-quantities-of-yttrium-oxide-were-image340223404.html
Observations on the rare earths : yttrium chloride and the atomic weight of yttrium . d tofractionation of earth mixtures containing didymium or gadolinium unless theyare first removed by the potassium sulphate method. The determination of the ratio Y2O3 : Y2(S04.)3 as a means of finding theatomic weight of yttrium was studied and found to be rather unoertain. Themethod aprarently gives atomio weight values which are lower than those gain-ed by other more reliable methods. The ratio Ya03 : 2YC13 waR studied and some very consistent results wereobtained. Weighed quantities of yttrium oxide were Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/observations-on-the-rare-earths-yttrium-chloride-and-the-atomic-weight-of-yttrium-d-tofractionation-of-earth-mixtures-containing-didymium-or-gadolinium-unless-theyare-first-removed-by-the-potassium-sulphate-method-the-determination-of-the-ratio-y2o3-y2s043-as-a-means-of-finding-theatomic-weight-of-yttrium-was-studied-and-found-to-be-rather-unoertain-themethod-aprarently-gives-atomio-weight-values-which-are-lower-than-those-gain-ed-by-other-more-reliable-methods-the-ratio-ya03-2yc13-war-studied-and-some-very-consistent-results-wereobtained-weighed-quantities-of-yttrium-oxide-were-image340223404.htmlRM2ANEEBT–Observations on the rare earths : yttrium chloride and the atomic weight of yttrium . d tofractionation of earth mixtures containing didymium or gadolinium unless theyare first removed by the potassium sulphate method. The determination of the ratio Y2O3 : Y2(S04.)3 as a means of finding theatomic weight of yttrium was studied and found to be rather unoertain. Themethod aprarently gives atomio weight values which are lower than those gain-ed by other more reliable methods. The ratio Ya03 : 2YC13 waR studied and some very consistent results wereobtained. Weighed quantities of yttrium oxide were
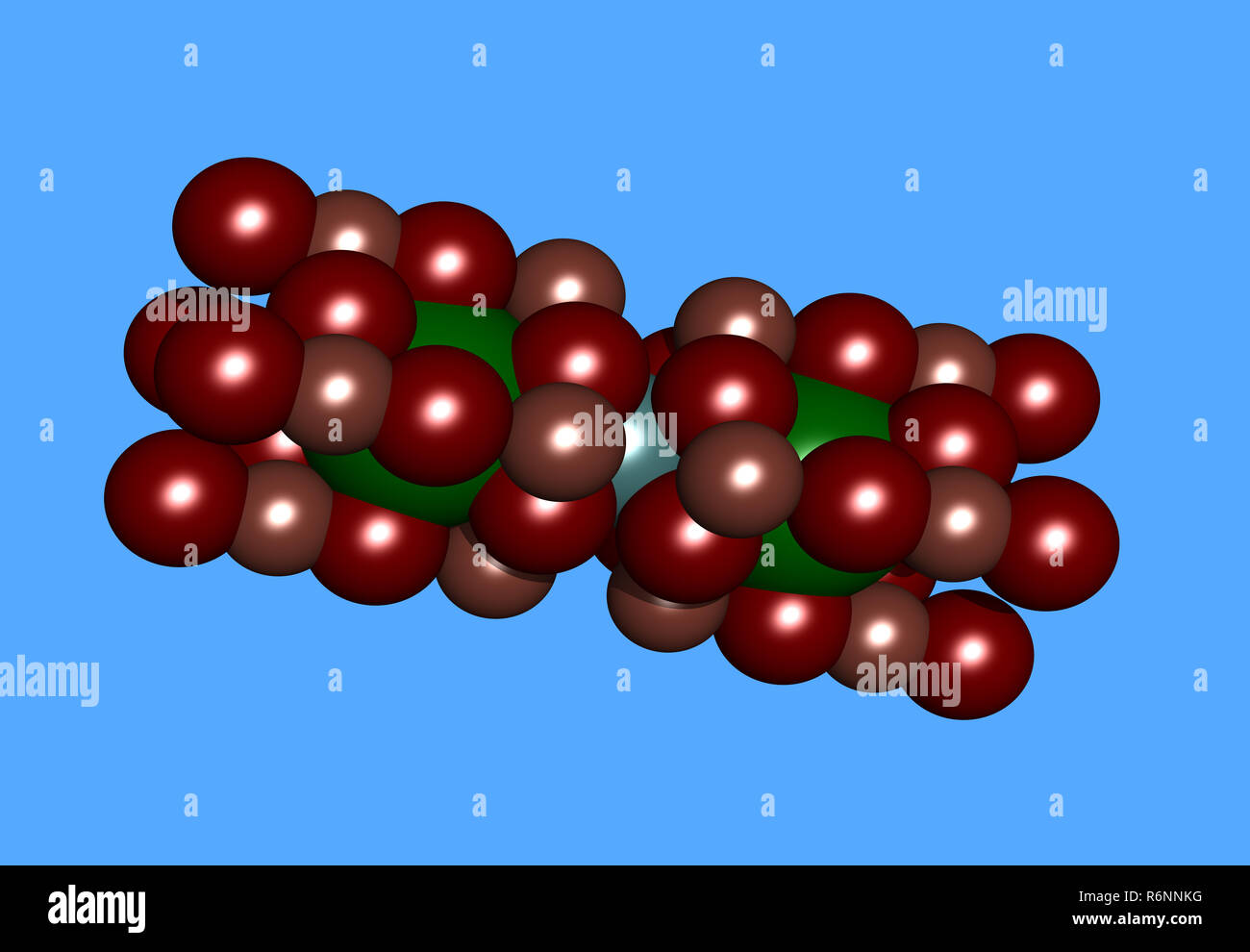 yttrium barium copper oxide molecular model Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/yttrium-barium-copper-oxide-molecular-model-image227900724.html
yttrium barium copper oxide molecular model Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/yttrium-barium-copper-oxide-molecular-model-image227900724.htmlRFR6NNKG–yttrium barium copper oxide molecular model
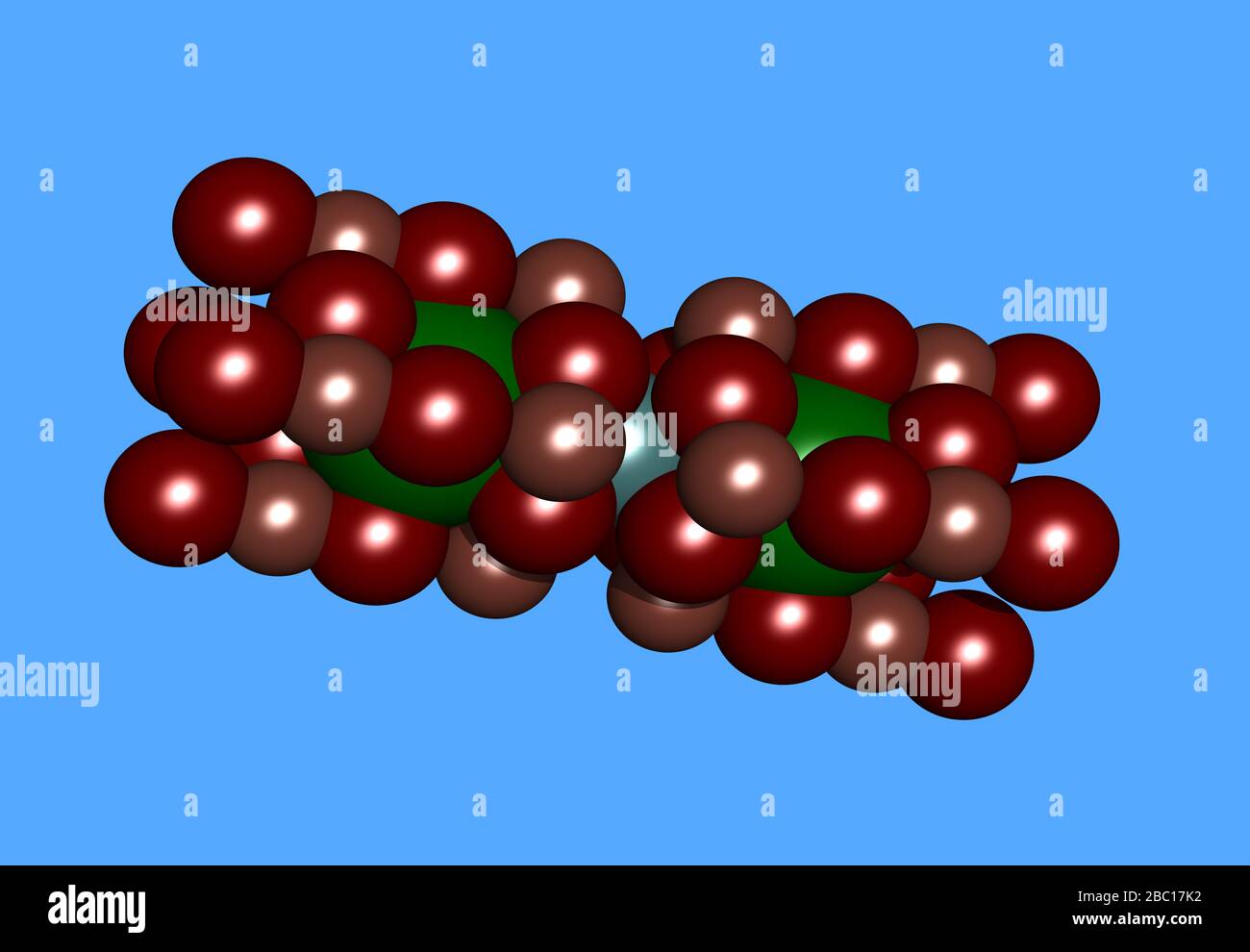 Yttrium Barium Kupfer Oxid molecular model with atoms Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/yttrium-barium-kupfer-oxid-molecular-model-with-atoms-image351611206.html
Yttrium Barium Kupfer Oxid molecular model with atoms Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/yttrium-barium-kupfer-oxid-molecular-model-with-atoms-image351611206.htmlRF2BC17K2–Yttrium Barium Kupfer Oxid molecular model with atoms
 Superconductivity Demonstation Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/superconductivity-demonstation-image352775882.html
Superconductivity Demonstation Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/superconductivity-demonstation-image352775882.htmlRM2BDX96J–Superconductivity Demonstation
 Meissner Effect, Levitation of Superconductor Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/stock-photo-meissner-effect-levitation-of-superconductor-135012064.html
Meissner Effect, Levitation of Superconductor Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/stock-photo-meissner-effect-levitation-of-superconductor-135012064.htmlRMHRJ97C–Meissner Effect, Levitation of Superconductor
 rare earth yttrium oxide, pictured at the Tradium GmbH in Frankfurt, Germany, Nov.5, 2012. Yttrium is used in making phosphors for television set CRT displays and in LEDs. Tradium is considered one of Europe's biggest traders in technology metals and rare Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/stock-photo-rare-earth-yttrium-oxide-pictured-at-the-tradium-gmbh-in-frankfurt-77085858.html
rare earth yttrium oxide, pictured at the Tradium GmbH in Frankfurt, Germany, Nov.5, 2012. Yttrium is used in making phosphors for television set CRT displays and in LEDs. Tradium is considered one of Europe's biggest traders in technology metals and rare Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/stock-photo-rare-earth-yttrium-oxide-pictured-at-the-tradium-gmbh-in-frankfurt-77085858.htmlRMEDBFPA–rare earth yttrium oxide, pictured at the Tradium GmbH in Frankfurt, Germany, Nov.5, 2012. Yttrium is used in making phosphors for television set CRT displays and in LEDs. Tradium is considered one of Europe's biggest traders in technology metals and rare
 Y2O3 yttria yttrium oxide CAS 1314-36-9 chemical substance in white plastic laboratory packaging Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/y2o3-yttria-yttrium-oxide-cas-1314-36-9-chemical-substance-in-white-plastic-laboratory-packaging-image542669084.html
Y2O3 yttria yttrium oxide CAS 1314-36-9 chemical substance in white plastic laboratory packaging Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/y2o3-yttria-yttrium-oxide-cas-1314-36-9-chemical-substance-in-white-plastic-laboratory-packaging-image542669084.htmlRF2PETKXM–Y2O3 yttria yttrium oxide CAS 1314-36-9 chemical substance in white plastic laboratory packaging
 Yttrium is a chemical element with the symbol Y and atomic number 39. Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/yttrium-is-a-chemical-element-with-the-symbol-y-and-atomic-number-39-image415993635.html
Yttrium is a chemical element with the symbol Y and atomic number 39. Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/yttrium-is-a-chemical-element-with-the-symbol-y-and-atomic-number-39-image415993635.htmlRM2F4P43F–Yttrium is a chemical element with the symbol Y and atomic number 39.
 . Selective radiation from various solids . ytoward the short wave-lengths, being at about i.8/x for an energyconsumption of 13.6 watts. The pure oxide is not an efficientradiator of white light, and only becomes so when a small amountof cerium, thorium, or yttrium oxide is added, which combinationis the Nernst glower previously investigated.^ In addition to the sharp emission lines at 2.8 and 4.35/>t, thereare wide hazy bands at 2 and 2.4ft (appears on curve d), while from5 to 6fjL there is a wide band which is evidently unresolved, themaximum shifting toward the short wave-lengths with ri Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/selective-radiation-from-various-solids-ytoward-the-short-wave-lengths-being-at-about-i8x-for-an-energyconsumption-of-136-watts-the-pure-oxide-is-not-an-efficientradiator-of-white-light-and-only-becomes-so-when-a-small-amountof-cerium-thorium-or-yttrium-oxide-is-added-which-combinationis-the-nernst-glower-previously-investigated-in-addition-to-the-sharp-emission-lines-at-28-and-435gtt-thereare-wide-hazy-bands-at-2-and-24ft-appears-on-curve-d-while-from5-to-6fjl-there-is-a-wide-band-which-is-evidently-unresolved-themaximum-shifting-toward-the-short-wave-lengths-with-ri-image370190946.html
. Selective radiation from various solids . ytoward the short wave-lengths, being at about i.8/x for an energyconsumption of 13.6 watts. The pure oxide is not an efficientradiator of white light, and only becomes so when a small amountof cerium, thorium, or yttrium oxide is added, which combinationis the Nernst glower previously investigated.^ In addition to the sharp emission lines at 2.8 and 4.35/>t, thereare wide hazy bands at 2 and 2.4ft (appears on curve d), while from5 to 6fjL there is a wide band which is evidently unresolved, themaximum shifting toward the short wave-lengths with ri Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/selective-radiation-from-various-solids-ytoward-the-short-wave-lengths-being-at-about-i8x-for-an-energyconsumption-of-136-watts-the-pure-oxide-is-not-an-efficientradiator-of-white-light-and-only-becomes-so-when-a-small-amountof-cerium-thorium-or-yttrium-oxide-is-added-which-combinationis-the-nernst-glower-previously-investigated-in-addition-to-the-sharp-emission-lines-at-28-and-435gtt-thereare-wide-hazy-bands-at-2-and-24ft-appears-on-curve-d-while-from5-to-6fjl-there-is-a-wide-band-which-is-evidently-unresolved-themaximum-shifting-toward-the-short-wave-lengths-with-ri-image370190946.htmlRM2CE7J96–. Selective radiation from various solids . ytoward the short wave-lengths, being at about i.8/x for an energyconsumption of 13.6 watts. The pure oxide is not an efficientradiator of white light, and only becomes so when a small amountof cerium, thorium, or yttrium oxide is added, which combinationis the Nernst glower previously investigated.^ In addition to the sharp emission lines at 2.8 and 4.35/>t, thereare wide hazy bands at 2 and 2.4ft (appears on curve d), while from5 to 6fjL there is a wide band which is evidently unresolved, themaximum shifting toward the short wave-lengths with ri
 Rare earths ceroxide, yttrium oxide and neodymium oxide, photographed at the Tradium GmbH in Frankfurt, Germany, Nov.5, 2012. Tradium is considered one of Europe's biggest traders in technology metals and rare earths. Photo: Frank Rumpenhorst/dpa Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/stock-photo-rare-earths-ceroxide-yttrium-oxide-and-neodymium-oxide-photographed-77088093.html
Rare earths ceroxide, yttrium oxide and neodymium oxide, photographed at the Tradium GmbH in Frankfurt, Germany, Nov.5, 2012. Tradium is considered one of Europe's biggest traders in technology metals and rare earths. Photo: Frank Rumpenhorst/dpa Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/stock-photo-rare-earths-ceroxide-yttrium-oxide-and-neodymium-oxide-photographed-77088093.htmlRMEDBJJ5–Rare earths ceroxide, yttrium oxide and neodymium oxide, photographed at the Tradium GmbH in Frankfurt, Germany, Nov.5, 2012. Tradium is considered one of Europe's biggest traders in technology metals and rare earths. Photo: Frank Rumpenhorst/dpa
 Yttrium is a chemical element with the symbol Y and atomic number 39. Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/yttrium-is-a-chemical-element-with-the-symbol-y-and-atomic-number-39-image415993721.html
Yttrium is a chemical element with the symbol Y and atomic number 39. Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/yttrium-is-a-chemical-element-with-the-symbol-y-and-atomic-number-39-image415993721.htmlRM2F4P46H–Yttrium is a chemical element with the symbol Y and atomic number 39.
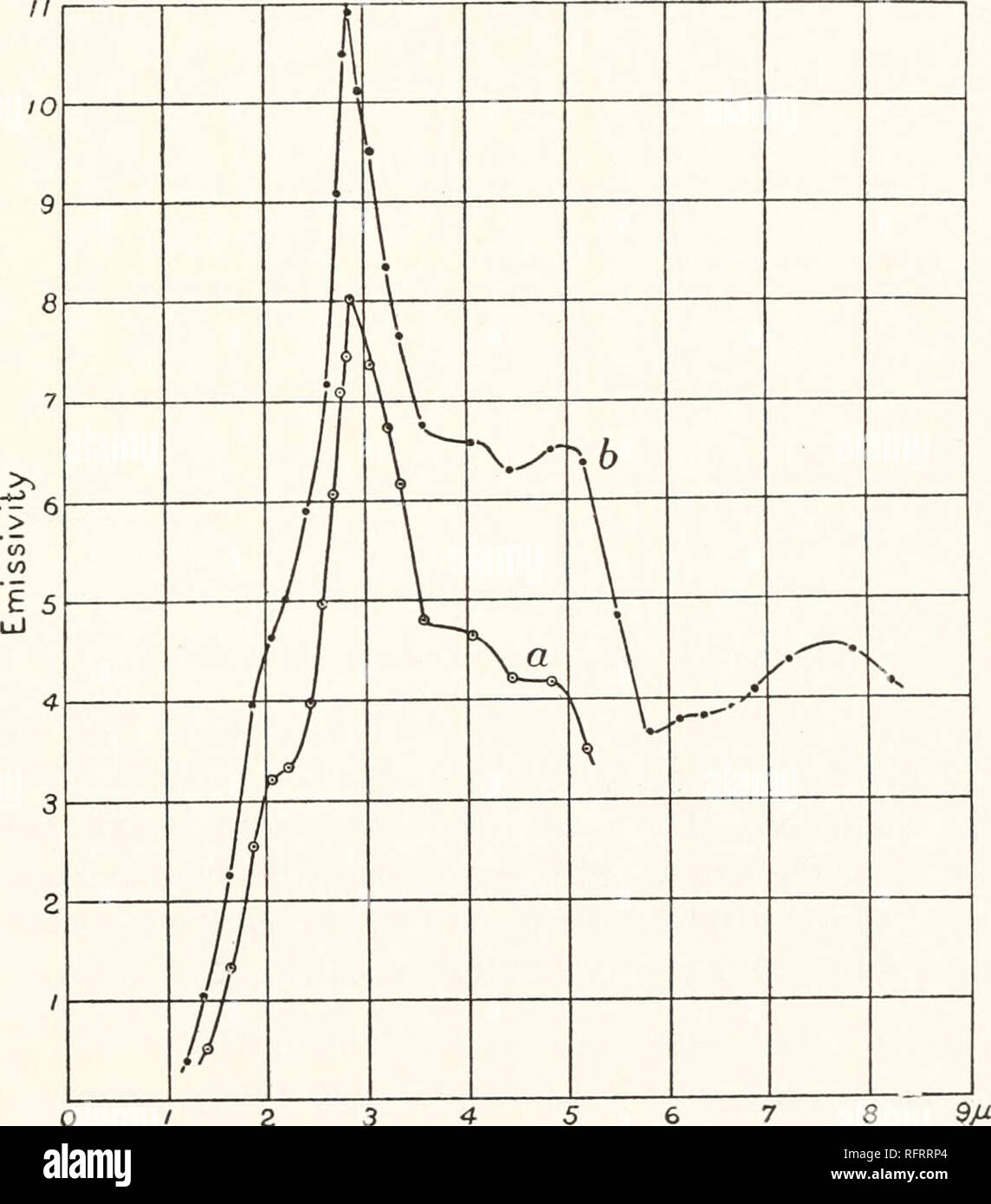 . Carnegie Institution of Washington publication. ERBIUM OXIDE. 117 yttrium oxide, of unknown purity, all of which were a beautiful yellow color, as compared with the sample given in fig. 83, which was a yellowish- white. These samples were obtained by fractional precipitation from the sulphate of yttrium by means of oxalic acid. The precipitation, however, was not carried out to the extent of obtaining yttrium, erbium, and ytter- bium oxides separately. In these curves the prominent bands of fig. 83, with maxima at 2 and 2.75 fi, are suppressed, and the small bands of the latter at 3 and 6.8 Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/carnegie-institution-of-washington-publication-erbium-oxide-117-yttrium-oxide-of-unknown-purity-all-of-which-were-a-beautiful-yellow-color-as-compared-with-the-sample-given-in-fig-83-which-was-a-yellowish-white-these-samples-were-obtained-by-fractional-precipitation-from-the-sulphate-of-yttrium-by-means-of-oxalic-acid-the-precipitation-however-was-not-carried-out-to-the-extent-of-obtaining-yttrium-erbium-and-ytter-bium-oxides-separately-in-these-curves-the-prominent-bands-of-fig-83-with-maxima-at-2-and-275-fi-are-suppressed-and-the-small-bands-of-the-latter-at-3-and-68-image233478172.html
. Carnegie Institution of Washington publication. ERBIUM OXIDE. 117 yttrium oxide, of unknown purity, all of which were a beautiful yellow color, as compared with the sample given in fig. 83, which was a yellowish- white. These samples were obtained by fractional precipitation from the sulphate of yttrium by means of oxalic acid. The precipitation, however, was not carried out to the extent of obtaining yttrium, erbium, and ytter- bium oxides separately. In these curves the prominent bands of fig. 83, with maxima at 2 and 2.75 fi, are suppressed, and the small bands of the latter at 3 and 6.8 Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/carnegie-institution-of-washington-publication-erbium-oxide-117-yttrium-oxide-of-unknown-purity-all-of-which-were-a-beautiful-yellow-color-as-compared-with-the-sample-given-in-fig-83-which-was-a-yellowish-white-these-samples-were-obtained-by-fractional-precipitation-from-the-sulphate-of-yttrium-by-means-of-oxalic-acid-the-precipitation-however-was-not-carried-out-to-the-extent-of-obtaining-yttrium-erbium-and-ytter-bium-oxides-separately-in-these-curves-the-prominent-bands-of-fig-83-with-maxima-at-2-and-275-fi-are-suppressed-and-the-small-bands-of-the-latter-at-3-and-68-image233478172.htmlRMRFRRP4–. Carnegie Institution of Washington publication. ERBIUM OXIDE. 117 yttrium oxide, of unknown purity, all of which were a beautiful yellow color, as compared with the sample given in fig. 83, which was a yellowish- white. These samples were obtained by fractional precipitation from the sulphate of yttrium by means of oxalic acid. The precipitation, however, was not carried out to the extent of obtaining yttrium, erbium, and ytter- bium oxides separately. In these curves the prominent bands of fig. 83, with maxima at 2 and 2.75 fi, are suppressed, and the small bands of the latter at 3 and 6.8
 Rare earths cer oxide, yttrium oxide and neodymium oxide are seen at the Tradium GmbH in Frankfurt, Germany, Nov.5, 2012. Tradium is considered one of Europe's biggest traders in technology metals and rare earths. Photo: Frank Rumpenhorst/dpa Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/stock-photo-rare-earths-cer-oxide-yttrium-oxide-and-neodymium-oxide-are-seen-at-77088143.html
Rare earths cer oxide, yttrium oxide and neodymium oxide are seen at the Tradium GmbH in Frankfurt, Germany, Nov.5, 2012. Tradium is considered one of Europe's biggest traders in technology metals and rare earths. Photo: Frank Rumpenhorst/dpa Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/stock-photo-rare-earths-cer-oxide-yttrium-oxide-and-neodymium-oxide-are-seen-at-77088143.htmlRMEDBJKY–Rare earths cer oxide, yttrium oxide and neodymium oxide are seen at the Tradium GmbH in Frankfurt, Germany, Nov.5, 2012. Tradium is considered one of Europe's biggest traders in technology metals and rare earths. Photo: Frank Rumpenhorst/dpa
 . Carnegie Institution of Washington publication. n6 INFRA-RED EMISSION SPECTRA. Yttrium Oxide (Y203). (Curves a and b, fig. 83; curves a, b, c, fig. 84.) The surface color in the two cases (fig. 83) was a deep and a bright red, corresponding to a temperature of 900° to iooo0. The two curves are similar in appearance, showing emission maxima at 2, 2.76, 3, 3.6, 4.6, and 6.9 ft, respectively, the latter band being unusually sharp. It will be 70. 4 5 Yttrium oxide. shown presently, in fig. 92, that this sharp band may be due to a suppres- sion of the radiation at 6 fi, caused by a band of metall Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/carnegie-institution-of-washington-publication-n6-infra-red-emission-spectra-yttrium-oxide-y203-curves-a-and-b-fig-83-curves-a-b-c-fig-84-the-surface-color-in-the-two-cases-fig-83-was-a-deep-and-a-bright-red-corresponding-to-a-temperature-of-900-to-iooo0-the-two-curves-are-similar-in-appearance-showing-emission-maxima-at-2-276-3-36-46-and-69-ft-respectively-the-latter-band-being-unusually-sharp-it-will-be-70-4-5-yttrium-oxide-shown-presently-in-fig-92-that-this-sharp-band-may-be-due-to-a-suppres-sion-of-the-radiation-at-6-fi-caused-by-a-band-of-metall-image233478178.html
. Carnegie Institution of Washington publication. n6 INFRA-RED EMISSION SPECTRA. Yttrium Oxide (Y203). (Curves a and b, fig. 83; curves a, b, c, fig. 84.) The surface color in the two cases (fig. 83) was a deep and a bright red, corresponding to a temperature of 900° to iooo0. The two curves are similar in appearance, showing emission maxima at 2, 2.76, 3, 3.6, 4.6, and 6.9 ft, respectively, the latter band being unusually sharp. It will be 70. 4 5 Yttrium oxide. shown presently, in fig. 92, that this sharp band may be due to a suppres- sion of the radiation at 6 fi, caused by a band of metall Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/carnegie-institution-of-washington-publication-n6-infra-red-emission-spectra-yttrium-oxide-y203-curves-a-and-b-fig-83-curves-a-b-c-fig-84-the-surface-color-in-the-two-cases-fig-83-was-a-deep-and-a-bright-red-corresponding-to-a-temperature-of-900-to-iooo0-the-two-curves-are-similar-in-appearance-showing-emission-maxima-at-2-276-3-36-46-and-69-ft-respectively-the-latter-band-being-unusually-sharp-it-will-be-70-4-5-yttrium-oxide-shown-presently-in-fig-92-that-this-sharp-band-may-be-due-to-a-suppres-sion-of-the-radiation-at-6-fi-caused-by-a-band-of-metall-image233478178.htmlRMRFRRPA–. Carnegie Institution of Washington publication. n6 INFRA-RED EMISSION SPECTRA. Yttrium Oxide (Y203). (Curves a and b, fig. 83; curves a, b, c, fig. 84.) The surface color in the two cases (fig. 83) was a deep and a bright red, corresponding to a temperature of 900° to iooo0. The two curves are similar in appearance, showing emission maxima at 2, 2.76, 3, 3.6, 4.6, and 6.9 ft, respectively, the latter band being unusually sharp. It will be 70. 4 5 Yttrium oxide. shown presently, in fig. 92, that this sharp band may be due to a suppres- sion of the radiation at 6 fi, caused by a band of metall
 Rare earths cer oxide, yttrium oxide and neodymium oxide are seen at the Tradium GmbH in Frankfurt, Germany, Nov.5, 2012. Tradium is considered one of Europe's biggest traders in technology metals and rare earths. Photo: Frank Rumpenhorst/dpa Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/stock-photo-rare-earths-cer-oxide-yttrium-oxide-and-neodymium-oxide-are-seen-at-77088137.html
Rare earths cer oxide, yttrium oxide and neodymium oxide are seen at the Tradium GmbH in Frankfurt, Germany, Nov.5, 2012. Tradium is considered one of Europe's biggest traders in technology metals and rare earths. Photo: Frank Rumpenhorst/dpa Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/stock-photo-rare-earths-cer-oxide-yttrium-oxide-and-neodymium-oxide-are-seen-at-77088137.htmlRMEDBJKN–Rare earths cer oxide, yttrium oxide and neodymium oxide are seen at the Tradium GmbH in Frankfurt, Germany, Nov.5, 2012. Tradium is considered one of Europe's biggest traders in technology metals and rare earths. Photo: Frank Rumpenhorst/dpa
 Observations on the rare earths : yttrium chloride and the atomic weight of yttrium . the lower numbers being the grams per fraction. From these results it may be concluded that the method serves very wellwhen fairly pure yttria is used as a starting material. Small quantities ofErbium are easily removed and also some very pure yttria may be separated froma mixture containing holmium. However, the method does not give good resultsfrom mixtures containing earths of the oerium group. The method used in the determination of the atomic weights was the con-version of weighed quantities of oxide to Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/observations-on-the-rare-earths-yttrium-chloride-and-the-atomic-weight-of-yttrium-the-lower-numbers-being-the-grams-per-fraction-from-these-results-it-may-be-concluded-that-the-method-serves-very-wellwhen-fairly-pure-yttria-is-used-as-a-starting-material-small-quantities-oferbium-are-easily-removed-and-also-some-very-pure-yttria-may-be-separated-froma-mixture-containing-holmium-however-the-method-does-not-give-good-resultsfrom-mixtures-containing-earths-of-the-oerium-group-the-method-used-in-the-determination-of-the-atomic-weights-was-the-con-version-of-weighed-quantities-of-oxide-to-image340227587.html
Observations on the rare earths : yttrium chloride and the atomic weight of yttrium . the lower numbers being the grams per fraction. From these results it may be concluded that the method serves very wellwhen fairly pure yttria is used as a starting material. Small quantities ofErbium are easily removed and also some very pure yttria may be separated froma mixture containing holmium. However, the method does not give good resultsfrom mixtures containing earths of the oerium group. The method used in the determination of the atomic weights was the con-version of weighed quantities of oxide to Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/observations-on-the-rare-earths-yttrium-chloride-and-the-atomic-weight-of-yttrium-the-lower-numbers-being-the-grams-per-fraction-from-these-results-it-may-be-concluded-that-the-method-serves-very-wellwhen-fairly-pure-yttria-is-used-as-a-starting-material-small-quantities-oferbium-are-easily-removed-and-also-some-very-pure-yttria-may-be-separated-froma-mixture-containing-holmium-however-the-method-does-not-give-good-resultsfrom-mixtures-containing-earths-of-the-oerium-group-the-method-used-in-the-determination-of-the-atomic-weights-was-the-con-version-of-weighed-quantities-of-oxide-to-image340227587.htmlRM2ANEKN7–Observations on the rare earths : yttrium chloride and the atomic weight of yttrium . the lower numbers being the grams per fraction. From these results it may be concluded that the method serves very wellwhen fairly pure yttria is used as a starting material. Small quantities ofErbium are easily removed and also some very pure yttria may be separated froma mixture containing holmium. However, the method does not give good resultsfrom mixtures containing earths of the oerium group. The method used in the determination of the atomic weights was the con-version of weighed quantities of oxide to
 Rare earths cer oxide, yttrium oxide and neodymium oxide are seen at the Tradium GmbH in Frankfurt, Germany, Nov.5, 2012. Tradium is considered one of Europe's biggest traders in technology metals and rare earths. Photo: Frank Rumpenhorst/dpa Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/stock-photo-rare-earths-cer-oxide-yttrium-oxide-and-neodymium-oxide-are-seen-at-77088112.html
Rare earths cer oxide, yttrium oxide and neodymium oxide are seen at the Tradium GmbH in Frankfurt, Germany, Nov.5, 2012. Tradium is considered one of Europe's biggest traders in technology metals and rare earths. Photo: Frank Rumpenhorst/dpa Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/stock-photo-rare-earths-cer-oxide-yttrium-oxide-and-neodymium-oxide-are-seen-at-77088112.htmlRMEDBJJT–Rare earths cer oxide, yttrium oxide and neodymium oxide are seen at the Tradium GmbH in Frankfurt, Germany, Nov.5, 2012. Tradium is considered one of Europe's biggest traders in technology metals and rare earths. Photo: Frank Rumpenhorst/dpa
 Observations on the rare earths : yttrium chloride and the atomic weight of yttrium . -11- IX. Gadolinite earth (average at. wt. 91.2)^46 grama of a jure white oxidewhioh had been obtained from bromate fraotions of gadolinite material andwhich had also been fractionated by the magnesium oxide and the potassium dou-ble sulphate methods were mixed with 165 grams of chromio aoid and fractionat-ed by the use of 250 oo. of ohrorcate solution per fraotion. A y11rium-didymiurn earth was also tried but no successful separation wasattained. Although the didymium did oonoentrate somewhat in the first fr Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/observations-on-the-rare-earths-yttrium-chloride-and-the-atomic-weight-of-yttrium-11-ix-gadolinite-earth-average-at-wt-91246-grama-of-a-jure-white-oxidewhioh-had-been-obtained-from-bromate-fraotions-of-gadolinite-material-andwhich-had-also-been-fractionated-by-the-magnesium-oxide-and-the-potassium-dou-ble-sulphate-methods-were-mixed-with-165-grams-of-chromio-aoid-and-fractionat-ed-by-the-use-of-250-oo-of-ohrorcate-solution-per-fraotion-a-y11rium-didymiurn-earth-was-also-tried-but-no-successful-separation-wasattained-although-the-didymium-did-oonoentrate-somewhat-in-the-first-fr-image340231201.html
Observations on the rare earths : yttrium chloride and the atomic weight of yttrium . -11- IX. Gadolinite earth (average at. wt. 91.2)^46 grama of a jure white oxidewhioh had been obtained from bromate fraotions of gadolinite material andwhich had also been fractionated by the magnesium oxide and the potassium dou-ble sulphate methods were mixed with 165 grams of chromio aoid and fractionat-ed by the use of 250 oo. of ohrorcate solution per fraotion. A y11rium-didymiurn earth was also tried but no successful separation wasattained. Although the didymium did oonoentrate somewhat in the first fr Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/observations-on-the-rare-earths-yttrium-chloride-and-the-atomic-weight-of-yttrium-11-ix-gadolinite-earth-average-at-wt-91246-grama-of-a-jure-white-oxidewhioh-had-been-obtained-from-bromate-fraotions-of-gadolinite-material-andwhich-had-also-been-fractionated-by-the-magnesium-oxide-and-the-potassium-dou-ble-sulphate-methods-were-mixed-with-165-grams-of-chromio-aoid-and-fractionat-ed-by-the-use-of-250-oo-of-ohrorcate-solution-per-fraotion-a-y11rium-didymiurn-earth-was-also-tried-but-no-successful-separation-wasattained-although-the-didymium-did-oonoentrate-somewhat-in-the-first-fr-image340231201.htmlRM2ANETA9–Observations on the rare earths : yttrium chloride and the atomic weight of yttrium . -11- IX. Gadolinite earth (average at. wt. 91.2)^46 grama of a jure white oxidewhioh had been obtained from bromate fraotions of gadolinite material andwhich had also been fractionated by the magnesium oxide and the potassium dou-ble sulphate methods were mixed with 165 grams of chromio aoid and fractionat-ed by the use of 250 oo. of ohrorcate solution per fraotion. A y11rium-didymiurn earth was also tried but no successful separation wasattained. Although the didymium did oonoentrate somewhat in the first fr
 rare earth yttrium oxide, pictured at the Tradium GmbH in Frankfurt, Germany, Nov.5, 2012. Yttrium is used in making phosphors for television set CRT displays and in LEDs. Tradium is considered one of Europe's biggest traders in technology metals and rare Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/stock-photo-rare-earth-yttrium-oxide-pictured-at-the-tradium-gmbh-in-frankfurt-77088127.html
rare earth yttrium oxide, pictured at the Tradium GmbH in Frankfurt, Germany, Nov.5, 2012. Yttrium is used in making phosphors for television set CRT displays and in LEDs. Tradium is considered one of Europe's biggest traders in technology metals and rare Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/stock-photo-rare-earth-yttrium-oxide-pictured-at-the-tradium-gmbh-in-frankfurt-77088127.htmlRMEDBJKB–rare earth yttrium oxide, pictured at the Tradium GmbH in Frankfurt, Germany, Nov.5, 2012. Yttrium is used in making phosphors for television set CRT displays and in LEDs. Tradium is considered one of Europe's biggest traders in technology metals and rare
 Observations on the rare earths : yttrium chloride and the atomic weight of yttrium . -11- IX. Gadolinite earth (average at. wt. 91.2)^46 grama of a jure white oxidewhioh had been obtained from bromate fraotions of gadolinite material andwhich had also been fractionated by the magnesium oxide and the potassium dou-ble sulphate methods were mixed with 165 grams of chromio aoid and fractionat-ed by the use of 250 oo. of ohrorcate solution per fraotion. A y11rium-didymiurn earth was also tried but no successful separation wasattained. Although the didymium did oonoentrate somewhat in the first fr Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/observations-on-the-rare-earths-yttrium-chloride-and-the-atomic-weight-of-yttrium-11-ix-gadolinite-earth-average-at-wt-91246-grama-of-a-jure-white-oxidewhioh-had-been-obtained-from-bromate-fraotions-of-gadolinite-material-andwhich-had-also-been-fractionated-by-the-magnesium-oxide-and-the-potassium-dou-ble-sulphate-methods-were-mixed-with-165-grams-of-chromio-aoid-and-fractionat-ed-by-the-use-of-250-oo-of-ohrorcate-solution-per-fraotion-a-y11rium-didymiurn-earth-was-also-tried-but-no-successful-separation-wasattained-although-the-didymium-did-oonoentrate-somewhat-in-the-first-fr-image340227806.html
Observations on the rare earths : yttrium chloride and the atomic weight of yttrium . -11- IX. Gadolinite earth (average at. wt. 91.2)^46 grama of a jure white oxidewhioh had been obtained from bromate fraotions of gadolinite material andwhich had also been fractionated by the magnesium oxide and the potassium dou-ble sulphate methods were mixed with 165 grams of chromio aoid and fractionat-ed by the use of 250 oo. of ohrorcate solution per fraotion. A y11rium-didymiurn earth was also tried but no successful separation wasattained. Although the didymium did oonoentrate somewhat in the first fr Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/observations-on-the-rare-earths-yttrium-chloride-and-the-atomic-weight-of-yttrium-11-ix-gadolinite-earth-average-at-wt-91246-grama-of-a-jure-white-oxidewhioh-had-been-obtained-from-bromate-fraotions-of-gadolinite-material-andwhich-had-also-been-fractionated-by-the-magnesium-oxide-and-the-potassium-dou-ble-sulphate-methods-were-mixed-with-165-grams-of-chromio-aoid-and-fractionat-ed-by-the-use-of-250-oo-of-ohrorcate-solution-per-fraotion-a-y11rium-didymiurn-earth-was-also-tried-but-no-successful-separation-wasattained-although-the-didymium-did-oonoentrate-somewhat-in-the-first-fr-image340227806.htmlRM2ANEM12–Observations on the rare earths : yttrium chloride and the atomic weight of yttrium . -11- IX. Gadolinite earth (average at. wt. 91.2)^46 grama of a jure white oxidewhioh had been obtained from bromate fraotions of gadolinite material andwhich had also been fractionated by the magnesium oxide and the potassium dou-ble sulphate methods were mixed with 165 grams of chromio aoid and fractionat-ed by the use of 250 oo. of ohrorcate solution per fraotion. A y11rium-didymiurn earth was also tried but no successful separation wasattained. Although the didymium did oonoentrate somewhat in the first fr
 rare earth yttrium oxide, pictured at the Tradium GmbH in Frankfurt, Germany, Nov.5, 2012. Yttrium is used in making phosphors for television set CRT displays and in LEDs. Tradium is considered one of Europe's biggest traders in technology metals and rare Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/stock-photo-rare-earth-yttrium-oxide-pictured-at-the-tradium-gmbh-in-frankfurt-77088119.html
rare earth yttrium oxide, pictured at the Tradium GmbH in Frankfurt, Germany, Nov.5, 2012. Yttrium is used in making phosphors for television set CRT displays and in LEDs. Tradium is considered one of Europe's biggest traders in technology metals and rare Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/stock-photo-rare-earth-yttrium-oxide-pictured-at-the-tradium-gmbh-in-frankfurt-77088119.htmlRMEDBJK3–rare earth yttrium oxide, pictured at the Tradium GmbH in Frankfurt, Germany, Nov.5, 2012. Yttrium is used in making phosphors for television set CRT displays and in LEDs. Tradium is considered one of Europe's biggest traders in technology metals and rare
 Observations on the rare earths : yttrium chloride and the atomic weight of yttrium . he best material (VTIIn, VIIIi2,VHIig, VIII14, Vis and Vila) were oombined and purified as follows: The oxide was dissolved in pure oommeroial hydroohlorio acid and preoip-itated as oxalate with pure commercial oxalic acid and washed until all tracesof chromium were removed. After ignition in platinum dishes in an electricoven the oxide was again dissolved in hydroohlorio aoid and a portion of thesolution tested with hydrogen sulphide. No precipitate was formed so thesolution was diluted considerably and satu Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/observations-on-the-rare-earths-yttrium-chloride-and-the-atomic-weight-of-yttrium-he-best-material-vtiin-viiii2vhiig-viii14-vis-and-vila-were-oombined-and-purified-as-follows-the-oxide-was-dissolved-in-pure-oommeroial-hydroohlorio-acid-and-preoip-itated-as-oxalate-with-pure-commercial-oxalic-acid-and-washed-until-all-tracesof-chromium-were-removed-after-ignition-in-platinum-dishes-in-an-electricoven-the-oxide-was-again-dissolved-in-hydroohlorio-aoid-and-a-portion-of-thesolution-tested-with-hydrogen-sulphide-no-precipitate-was-formed-so-thesolution-was-diluted-considerably-and-satu-image340227403.html
Observations on the rare earths : yttrium chloride and the atomic weight of yttrium . he best material (VTIIn, VIIIi2,VHIig, VIII14, Vis and Vila) were oombined and purified as follows: The oxide was dissolved in pure oommeroial hydroohlorio acid and preoip-itated as oxalate with pure commercial oxalic acid and washed until all tracesof chromium were removed. After ignition in platinum dishes in an electricoven the oxide was again dissolved in hydroohlorio aoid and a portion of thesolution tested with hydrogen sulphide. No precipitate was formed so thesolution was diluted considerably and satu Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/observations-on-the-rare-earths-yttrium-chloride-and-the-atomic-weight-of-yttrium-he-best-material-vtiin-viiii2vhiig-viii14-vis-and-vila-were-oombined-and-purified-as-follows-the-oxide-was-dissolved-in-pure-oommeroial-hydroohlorio-acid-and-preoip-itated-as-oxalate-with-pure-commercial-oxalic-acid-and-washed-until-all-tracesof-chromium-were-removed-after-ignition-in-platinum-dishes-in-an-electricoven-the-oxide-was-again-dissolved-in-hydroohlorio-aoid-and-a-portion-of-thesolution-tested-with-hydrogen-sulphide-no-precipitate-was-formed-so-thesolution-was-diluted-considerably-and-satu-image340227403.htmlRM2ANEKEK–Observations on the rare earths : yttrium chloride and the atomic weight of yttrium . he best material (VTIIn, VIIIi2,VHIig, VIII14, Vis and Vila) were oombined and purified as follows: The oxide was dissolved in pure oommeroial hydroohlorio acid and preoip-itated as oxalate with pure commercial oxalic acid and washed until all tracesof chromium were removed. After ignition in platinum dishes in an electricoven the oxide was again dissolved in hydroohlorio aoid and a portion of thesolution tested with hydrogen sulphide. No precipitate was formed so thesolution was diluted considerably and satu
 rare earth yttrium oxide, pictured at the Tradium GmbH in Frankfurt, Germany, Nov.5, 2012. Yttrium is used in making phosphors for television set CRT displays and in LEDs. Tradium is considered one of Europe's biggest traders in technology metals and rare Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/stock-photo-rare-earth-yttrium-oxide-pictured-at-the-tradium-gmbh-in-frankfurt-77085851.html
rare earth yttrium oxide, pictured at the Tradium GmbH in Frankfurt, Germany, Nov.5, 2012. Yttrium is used in making phosphors for television set CRT displays and in LEDs. Tradium is considered one of Europe's biggest traders in technology metals and rare Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/stock-photo-rare-earth-yttrium-oxide-pictured-at-the-tradium-gmbh-in-frankfurt-77085851.htmlRMEDBFP3–rare earth yttrium oxide, pictured at the Tradium GmbH in Frankfurt, Germany, Nov.5, 2012. Yttrium is used in making phosphors for television set CRT displays and in LEDs. Tradium is considered one of Europe's biggest traders in technology metals and rare
 Observations on the rare earths : yttrium chloride and the atomic weight of yttrium . ched. The following prooedure was tried. Small samples (generally .2 to .5 gr,oxide )were weighed in platinum oruoibles. The oxide was then oarefully moist-ened with water, and covered with pure hydroohlorio aoid, plaoed over a steambath, and proteoted from dust. When dissolved a slight excess of the calculat ed amount of dilute sulphuric aoid was added and evaporated to dryness. It o was then heated for four hours in an electric furnaoe at 500 , and weighed.To prevent absorption of moisture from air by the a Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/observations-on-the-rare-earths-yttrium-chloride-and-the-atomic-weight-of-yttrium-ched-the-following-prooedure-was-tried-small-samples-generally-2-to-5-groxide-were-weighed-in-platinum-oruoibles-the-oxide-was-then-oarefully-moist-ened-with-water-and-covered-with-pure-hydroohlorio-aoid-plaoed-over-a-steambath-and-proteoted-from-dust-when-dissolved-a-slight-excess-of-the-calculat-ed-amount-of-dilute-sulphuric-aoid-was-added-and-evaporated-to-dryness-it-o-was-then-heated-for-four-hours-in-an-electric-furnaoe-at-500-and-weighedto-prevent-absorption-of-moisture-from-air-by-the-a-image340227068.html
Observations on the rare earths : yttrium chloride and the atomic weight of yttrium . ched. The following prooedure was tried. Small samples (generally .2 to .5 gr,oxide )were weighed in platinum oruoibles. The oxide was then oarefully moist-ened with water, and covered with pure hydroohlorio aoid, plaoed over a steambath, and proteoted from dust. When dissolved a slight excess of the calculat ed amount of dilute sulphuric aoid was added and evaporated to dryness. It o was then heated for four hours in an electric furnaoe at 500 , and weighed.To prevent absorption of moisture from air by the a Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/observations-on-the-rare-earths-yttrium-chloride-and-the-atomic-weight-of-yttrium-ched-the-following-prooedure-was-tried-small-samples-generally-2-to-5-groxide-were-weighed-in-platinum-oruoibles-the-oxide-was-then-oarefully-moist-ened-with-water-and-covered-with-pure-hydroohlorio-aoid-plaoed-over-a-steambath-and-proteoted-from-dust-when-dissolved-a-slight-excess-of-the-calculat-ed-amount-of-dilute-sulphuric-aoid-was-added-and-evaporated-to-dryness-it-o-was-then-heated-for-four-hours-in-an-electric-furnaoe-at-500-and-weighedto-prevent-absorption-of-moisture-from-air-by-the-a-image340227068.htmlRM2ANEK2M–Observations on the rare earths : yttrium chloride and the atomic weight of yttrium . ched. The following prooedure was tried. Small samples (generally .2 to .5 gr,oxide )were weighed in platinum oruoibles. The oxide was then oarefully moist-ened with water, and covered with pure hydroohlorio aoid, plaoed over a steambath, and proteoted from dust. When dissolved a slight excess of the calculat ed amount of dilute sulphuric aoid was added and evaporated to dryness. It o was then heated for four hours in an electric furnaoe at 500 , and weighed.To prevent absorption of moisture from air by the a
 rare earth yttrium oxide, pictured at the Tradium GmbH in Frankfurt, Germany, Nov.5, 2012. Yttrium is used in making phosphors for television set CRT displays and in LEDs. Tradium is considered one of Europe's biggest traders in technology metals and rare Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/stock-photo-rare-earth-yttrium-oxide-pictured-at-the-tradium-gmbh-in-frankfurt-77088101.html
rare earth yttrium oxide, pictured at the Tradium GmbH in Frankfurt, Germany, Nov.5, 2012. Yttrium is used in making phosphors for television set CRT displays and in LEDs. Tradium is considered one of Europe's biggest traders in technology metals and rare Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/stock-photo-rare-earth-yttrium-oxide-pictured-at-the-tradium-gmbh-in-frankfurt-77088101.htmlRMEDBJJD–rare earth yttrium oxide, pictured at the Tradium GmbH in Frankfurt, Germany, Nov.5, 2012. Yttrium is used in making phosphors for television set CRT displays and in LEDs. Tradium is considered one of Europe's biggest traders in technology metals and rare
 Observations on the rare earths : yttrium chloride and the atomic weight of yttrium . thenitrate solution and then while the solution was being rapidly stirred, potas-sium permanganate solution was added slowly until after oontinued stirringthe liquid retained a red oolor. A little oerium was still left in the solu-t ion. However an objection to the use of zino oxide in the above method lay inthe faot that a considerable quantity of zino remained in solution and wanoarried down to a large per oent. when the remaining earths were preoipitatedwith oxalio acid. For this reason the use of magnesiu Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/observations-on-the-rare-earths-yttrium-chloride-and-the-atomic-weight-of-yttrium-thenitrate-solution-and-then-while-the-solution-was-being-rapidly-stirred-potas-sium-permanganate-solution-was-added-slowly-until-after-oontinued-stirringthe-liquid-retained-a-red-oolor-a-little-oerium-was-still-left-in-the-solu-t-ion-however-an-objection-to-the-use-of-zino-oxide-in-the-above-method-lay-inthe-faot-that-a-considerable-quantity-of-zino-remained-in-solution-and-wanoarried-down-to-a-large-per-oent-when-the-remaining-earths-were-preoipitatedwith-oxalio-acid-for-this-reason-the-use-of-magnesiu-image340233749.html
Observations on the rare earths : yttrium chloride and the atomic weight of yttrium . thenitrate solution and then while the solution was being rapidly stirred, potas-sium permanganate solution was added slowly until after oontinued stirringthe liquid retained a red oolor. A little oerium was still left in the solu-t ion. However an objection to the use of zino oxide in the above method lay inthe faot that a considerable quantity of zino remained in solution and wanoarried down to a large per oent. when the remaining earths were preoipitatedwith oxalio acid. For this reason the use of magnesiu Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/observations-on-the-rare-earths-yttrium-chloride-and-the-atomic-weight-of-yttrium-thenitrate-solution-and-then-while-the-solution-was-being-rapidly-stirred-potas-sium-permanganate-solution-was-added-slowly-until-after-oontinued-stirringthe-liquid-retained-a-red-oolor-a-little-oerium-was-still-left-in-the-solu-t-ion-however-an-objection-to-the-use-of-zino-oxide-in-the-above-method-lay-inthe-faot-that-a-considerable-quantity-of-zino-remained-in-solution-and-wanoarried-down-to-a-large-per-oent-when-the-remaining-earths-were-preoipitatedwith-oxalio-acid-for-this-reason-the-use-of-magnesiu-image340233749.htmlRM2ANEYH9–Observations on the rare earths : yttrium chloride and the atomic weight of yttrium . thenitrate solution and then while the solution was being rapidly stirred, potas-sium permanganate solution was added slowly until after oontinued stirringthe liquid retained a red oolor. A little oerium was still left in the solu-t ion. However an objection to the use of zino oxide in the above method lay inthe faot that a considerable quantity of zino remained in solution and wanoarried down to a large per oent. when the remaining earths were preoipitatedwith oxalio acid. For this reason the use of magnesiu
 Observations on the rare earths : yttrium chloride and the atomic weight of yttrium . •9- VII. Source of material was the same as for No. VI, Taut the process wascarried out according to a method suggested by Professor C. James in a pri-vate communication. 144- grams of oxide were dissolved in nitric acid to a neutral solution and so.ne potassium diohromate added, diluted to four with liters and fraotionated 500 cc. of ohromate solution as above.VIII. The following fractions from the above series were combined: FractionIllio IVeIVeIV7 Weight Atnmio Weight S grams 90.6 212621 75 149 90,490.290. Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/observations-on-the-rare-earths-yttrium-chloride-and-the-atomic-weight-of-yttrium-9-vii-source-of-material-was-the-same-as-for-no-vi-taut-the-process-wascarried-out-according-to-a-method-suggested-by-professor-c-james-in-a-pri-vate-communication-144-grams-of-oxide-were-dissolved-in-nitric-acid-to-a-neutral-solution-and-sone-potassium-diohromate-added-diluted-to-four-with-liters-and-fraotionated-500-cc-of-ohromate-solution-as-aboveviii-the-following-fractions-from-the-above-series-were-combined-fractionillio-iveiveiv7-weight-atnmio-weight-s-grams-906-212621-75-149-90490290-image340231433.html
Observations on the rare earths : yttrium chloride and the atomic weight of yttrium . •9- VII. Source of material was the same as for No. VI, Taut the process wascarried out according to a method suggested by Professor C. James in a pri-vate communication. 144- grams of oxide were dissolved in nitric acid to a neutral solution and so.ne potassium diohromate added, diluted to four with liters and fraotionated 500 cc. of ohromate solution as above.VIII. The following fractions from the above series were combined: FractionIllio IVeIVeIV7 Weight Atnmio Weight S grams 90.6 212621 75 149 90,490.290. Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/observations-on-the-rare-earths-yttrium-chloride-and-the-atomic-weight-of-yttrium-9-vii-source-of-material-was-the-same-as-for-no-vi-taut-the-process-wascarried-out-according-to-a-method-suggested-by-professor-c-james-in-a-pri-vate-communication-144-grams-of-oxide-were-dissolved-in-nitric-acid-to-a-neutral-solution-and-sone-potassium-diohromate-added-diluted-to-four-with-liters-and-fraotionated-500-cc-of-ohromate-solution-as-aboveviii-the-following-fractions-from-the-above-series-were-combined-fractionillio-iveiveiv7-weight-atnmio-weight-s-grams-906-212621-75-149-90490290-image340231433.htmlRM2ANETJH–Observations on the rare earths : yttrium chloride and the atomic weight of yttrium . •9- VII. Source of material was the same as for No. VI, Taut the process wascarried out according to a method suggested by Professor C. James in a pri-vate communication. 144- grams of oxide were dissolved in nitric acid to a neutral solution and so.ne potassium diohromate added, diluted to four with liters and fraotionated 500 cc. of ohromate solution as above.VIII. The following fractions from the above series were combined: FractionIllio IVeIVeIV7 Weight Atnmio Weight S grams 90.6 212621 75 149 90,490.290.
 Observations on the rare earths : yttrium chloride and the atomic weight of yttrium . 23.6) yttrium-hoImium fractionsfrom a brornate series. Oxalate white but oxide was light brown. Two 175 gramportions were taken and mixed with 370 grams ohrornic acid each. 500 co. ohromate solution wer? used in each fraction. IV. Yttrium-erbium earth (average at. wt. 92.5) from the brornate fraction-ation. 265 grams were mixed with double weight of ohromio aoid and dividedinto two flasks for the run. 500 oc. ohromate solution were used for eaohfract ion. V. Xenotime earth (average at. wt. 96.2), last fractio Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/observations-on-the-rare-earths-yttrium-chloride-and-the-atomic-weight-of-yttrium-236-yttrium-hoimium-fractionsfrom-a-brornate-series-oxalate-white-but-oxide-was-light-brown-two-175-gramportions-were-taken-and-mixed-with-370-grams-ohrornic-acid-each-500-co-ohromate-solution-wer-used-in-each-fraction-iv-yttrium-erbium-earth-average-at-wt-925-from-the-brornate-fraction-ation-265-grams-were-mixed-with-double-weight-of-ohromio-aoid-and-dividedinto-two-flasks-for-the-run-500-oc-ohromate-solution-were-used-for-eaohfract-ion-v-xenotime-earth-average-at-wt-962-last-fractio-image340231807.html
Observations on the rare earths : yttrium chloride and the atomic weight of yttrium . 23.6) yttrium-hoImium fractionsfrom a brornate series. Oxalate white but oxide was light brown. Two 175 gramportions were taken and mixed with 370 grams ohrornic acid each. 500 co. ohromate solution wer? used in each fraction. IV. Yttrium-erbium earth (average at. wt. 92.5) from the brornate fraction-ation. 265 grams were mixed with double weight of ohromio aoid and dividedinto two flasks for the run. 500 oc. ohromate solution were used for eaohfract ion. V. Xenotime earth (average at. wt. 96.2), last fractio Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/observations-on-the-rare-earths-yttrium-chloride-and-the-atomic-weight-of-yttrium-236-yttrium-hoimium-fractionsfrom-a-brornate-series-oxalate-white-but-oxide-was-light-brown-two-175-gramportions-were-taken-and-mixed-with-370-grams-ohrornic-acid-each-500-co-ohromate-solution-wer-used-in-each-fraction-iv-yttrium-erbium-earth-average-at-wt-925-from-the-brornate-fraction-ation-265-grams-were-mixed-with-double-weight-of-ohromio-aoid-and-dividedinto-two-flasks-for-the-run-500-oc-ohromate-solution-were-used-for-eaohfract-ion-v-xenotime-earth-average-at-wt-962-last-fractio-image340231807.htmlRM2ANEW3Y–Observations on the rare earths : yttrium chloride and the atomic weight of yttrium . 23.6) yttrium-hoImium fractionsfrom a brornate series. Oxalate white but oxide was light brown. Two 175 gramportions were taken and mixed with 370 grams ohrornic acid each. 500 co. ohromate solution wer? used in each fraction. IV. Yttrium-erbium earth (average at. wt. 92.5) from the brornate fraction-ation. 265 grams were mixed with double weight of ohromio aoid and dividedinto two flasks for the run. 500 oc. ohromate solution were used for eaohfract ion. V. Xenotime earth (average at. wt. 96.2), last fractio
 Observations on the rare earths : yttrium chloride and the atomic weight of yttrium . -14- orucibles containing the samples. The sulphates were finally treated withwater to determine if basic sulphate had been formed. The temperature of 0 0 0 Nos. 9 and 10 was held at 400 to 425 C instead of 500 C. The ratio used in calculating the atomio weights was: M + 24 wt. of oxide M + 144.105 wt. of sulphate, where M is the atomio weight desired. All weights were correoted to vaouun.. -15- TABLE II 0 b 16 Y203 ! Y2(S04)3 S = 32,07 No Weight ofOxide Weight ofSulphate AtomicWeight 1 1.00552 2.06854 89.61 Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/observations-on-the-rare-earths-yttrium-chloride-and-the-atomic-weight-of-yttrium-14-orucibles-containing-the-samples-the-sulphates-were-finally-treated-withwater-to-determine-if-basic-sulphate-had-been-formed-the-temperature-of-0-0-0-nos-9-and-10-was-held-at-400-to-425-c-instead-of-500-c-the-ratio-used-in-calculating-the-atomio-weights-was-m-24-wt-of-oxide-m-144105-wt-of-sulphate-where-m-is-the-atomio-weight-desired-all-weights-were-correoted-to-vaouun-15-table-ii-0-b-16-y203-!-y2s043-s-=-3207-no-weight-ofoxide-weight-ofsulphate-atomicweight-1-100552-206854-8961-image340226779.html
Observations on the rare earths : yttrium chloride and the atomic weight of yttrium . -14- orucibles containing the samples. The sulphates were finally treated withwater to determine if basic sulphate had been formed. The temperature of 0 0 0 Nos. 9 and 10 was held at 400 to 425 C instead of 500 C. The ratio used in calculating the atomio weights was: M + 24 wt. of oxide M + 144.105 wt. of sulphate, where M is the atomio weight desired. All weights were correoted to vaouun.. -15- TABLE II 0 b 16 Y203 ! Y2(S04)3 S = 32,07 No Weight ofOxide Weight ofSulphate AtomicWeight 1 1.00552 2.06854 89.61 Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/observations-on-the-rare-earths-yttrium-chloride-and-the-atomic-weight-of-yttrium-14-orucibles-containing-the-samples-the-sulphates-were-finally-treated-withwater-to-determine-if-basic-sulphate-had-been-formed-the-temperature-of-0-0-0-nos-9-and-10-was-held-at-400-to-425-c-instead-of-500-c-the-ratio-used-in-calculating-the-atomio-weights-was-m-24-wt-of-oxide-m-144105-wt-of-sulphate-where-m-is-the-atomio-weight-desired-all-weights-were-correoted-to-vaouun-15-table-ii-0-b-16-y203-!-y2s043-s-=-3207-no-weight-ofoxide-weight-ofsulphate-atomicweight-1-100552-206854-8961-image340226779.htmlRM2ANEJMB–Observations on the rare earths : yttrium chloride and the atomic weight of yttrium . -14- orucibles containing the samples. The sulphates were finally treated withwater to determine if basic sulphate had been formed. The temperature of 0 0 0 Nos. 9 and 10 was held at 400 to 425 C instead of 500 C. The ratio used in calculating the atomio weights was: M + 24 wt. of oxide M + 144.105 wt. of sulphate, where M is the atomio weight desired. All weights were correoted to vaouun.. -15- TABLE II 0 b 16 Y203 ! Y2(S04)3 S = 32,07 No Weight ofOxide Weight ofSulphate AtomicWeight 1 1.00552 2.06854 89.61
 Observations on the rare earths : yttrium chloride and the atomic weight of yttrium . -23- U + 24 - dteight of oxide ).{ + 1C5.38 Weight of chloride TA3LE III Y203 : 2YC13 n — i pi — OC Aft No. Weight of Weight of Atomio Weight Oxide Chloride 3 .72180 1.240 92 90.54 4 .80392 1.38437 90,10 •J 700*50 X . auoxu 6 .73030 1.25 755 90.11. -24- SUHrARY. Rare earth mixtures from Gadolinite and Xenotime were fraotionated byvariouR methods. The efficiency of the chromate method, as aprlied to vari-ous fractions from the bromate method, was studied. It was found to be arather rapid method for preparation Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/observations-on-the-rare-earths-yttrium-chloride-and-the-atomic-weight-of-yttrium-23-u-24-dteight-of-oxide-1c538-weight-of-chloride-ta3le-iii-y203-2yc13-n-i-pi-oc-aft-no-weight-of-weight-of-atomio-weight-oxide-chloride-3-72180-1240-92-9054-4-80392-138437-9010-j-70050-x-auoxu-6-73030-125-755-9011-24-suhrary-rare-earth-mixtures-from-gadolinite-and-xenotime-were-fraotionated-byvariour-methods-the-efficiency-of-the-chromate-method-as-aprlied-to-vari-ous-fractions-from-the-bromate-method-was-studied-it-was-found-to-be-arather-rapid-method-for-preparation-image340223627.html
Observations on the rare earths : yttrium chloride and the atomic weight of yttrium . -23- U + 24 - dteight of oxide ).{ + 1C5.38 Weight of chloride TA3LE III Y203 : 2YC13 n — i pi — OC Aft No. Weight of Weight of Atomio Weight Oxide Chloride 3 .72180 1.240 92 90.54 4 .80392 1.38437 90,10 •J 700*50 X . auoxu 6 .73030 1.25 755 90.11. -24- SUHrARY. Rare earth mixtures from Gadolinite and Xenotime were fraotionated byvariouR methods. The efficiency of the chromate method, as aprlied to vari-ous fractions from the bromate method, was studied. It was found to be arather rapid method for preparation Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/observations-on-the-rare-earths-yttrium-chloride-and-the-atomic-weight-of-yttrium-23-u-24-dteight-of-oxide-1c538-weight-of-chloride-ta3le-iii-y203-2yc13-n-i-pi-oc-aft-no-weight-of-weight-of-atomio-weight-oxide-chloride-3-72180-1240-92-9054-4-80392-138437-9010-j-70050-x-auoxu-6-73030-125-755-9011-24-suhrary-rare-earth-mixtures-from-gadolinite-and-xenotime-were-fraotionated-byvariour-methods-the-efficiency-of-the-chromate-method-as-aprlied-to-vari-ous-fractions-from-the-bromate-method-was-studied-it-was-found-to-be-arather-rapid-method-for-preparation-image340223627.htmlRM2ANEEKR–Observations on the rare earths : yttrium chloride and the atomic weight of yttrium . -23- U + 24 - dteight of oxide ).{ + 1C5.38 Weight of chloride TA3LE III Y203 : 2YC13 n — i pi — OC Aft No. Weight of Weight of Atomio Weight Oxide Chloride 3 .72180 1.240 92 90.54 4 .80392 1.38437 90,10 •J 700*50 X . auoxu 6 .73030 1.25 755 90.11. -24- SUHrARY. Rare earth mixtures from Gadolinite and Xenotime were fraotionated byvariouR methods. The efficiency of the chromate method, as aprlied to vari-ous fractions from the bromate method, was studied. It was found to be arather rapid method for preparation
RMRFRRPE–. Carnegie Institution of Washington publication. YTTRIUM OXIDES. 115 Beryllium Oxide (BeO); Silicon Oxide (Si02); Vanadium Oxide (V206). (Curve a=BeO; b and c = Si02; fig. 82.) The beryllium-oxide emission spectrum is smooth, with two wide maxima at 3.5 and 5 /*, respectively. The temperature was such that a faint red showed through the interstices of the layer of white oxide. The silicon-dioxide (quartz, French flint) curve shows bands at 2.2, 2-&3> 3-7> 4-4> and 5.3 /<, which coincide with the absorption bands (see Carnegie Publication No. 65, p. 21). For curve c the layer o
 . Carnegie Institution of Washington publication. n8 INFRA-RED EMISSION SPECTRA. tube. The two spectra are similar, with a sharp emission band at 2.85 [i. Other maxima appear at 2, 3.2, 4.1, 5, and 7.5 li. The general outline of the spectrum is similar to that of yttrium. Neodymium Oxide (NdO); Manganous Oxide (MnO). (Curve a, NdO; curve b, MnO; fig. 86.) The neodymium oxide was deposited in a thick layer upon a strip of platinum, by evaporation from a solution of the nitrate. The radiation curve shows maxima at 3, 4.4, and 4.83 ll. Beyond 6 ll the emissivity is strong and not unlike that of c Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/carnegie-institution-of-washington-publication-n8-infra-red-emission-spectra-tube-the-two-spectra-are-similar-with-a-sharp-emission-band-at-285-i-other-maxima-appear-at-2-32-41-5-and-75-li-the-general-outline-of-the-spectrum-is-similar-to-that-of-yttrium-neodymium-oxide-ndo-manganous-oxide-mno-curve-a-ndo-curve-b-mno-fig-86-the-neodymium-oxide-was-deposited-in-a-thick-layer-upon-a-strip-of-platinum-by-evaporation-from-a-solution-of-the-nitrate-the-radiation-curve-shows-maxima-at-3-44-and-483-ll-beyond-6-ll-the-emissivity-is-strong-and-not-unlike-that-of-c-image233478165.html
. Carnegie Institution of Washington publication. n8 INFRA-RED EMISSION SPECTRA. tube. The two spectra are similar, with a sharp emission band at 2.85 [i. Other maxima appear at 2, 3.2, 4.1, 5, and 7.5 li. The general outline of the spectrum is similar to that of yttrium. Neodymium Oxide (NdO); Manganous Oxide (MnO). (Curve a, NdO; curve b, MnO; fig. 86.) The neodymium oxide was deposited in a thick layer upon a strip of platinum, by evaporation from a solution of the nitrate. The radiation curve shows maxima at 3, 4.4, and 4.83 ll. Beyond 6 ll the emissivity is strong and not unlike that of c Stock Photohttps://www.alamy.com/image-license-details/?v=1https://www.alamy.com/carnegie-institution-of-washington-publication-n8-infra-red-emission-spectra-tube-the-two-spectra-are-similar-with-a-sharp-emission-band-at-285-i-other-maxima-appear-at-2-32-41-5-and-75-li-the-general-outline-of-the-spectrum-is-similar-to-that-of-yttrium-neodymium-oxide-ndo-manganous-oxide-mno-curve-a-ndo-curve-b-mno-fig-86-the-neodymium-oxide-was-deposited-in-a-thick-layer-upon-a-strip-of-platinum-by-evaporation-from-a-solution-of-the-nitrate-the-radiation-curve-shows-maxima-at-3-44-and-483-ll-beyond-6-ll-the-emissivity-is-strong-and-not-unlike-that-of-c-image233478165.htmlRMRFRRNW–. Carnegie Institution of Washington publication. n8 INFRA-RED EMISSION SPECTRA. tube. The two spectra are similar, with a sharp emission band at 2.85 [i. Other maxima appear at 2, 3.2, 4.1, 5, and 7.5 li. The general outline of the spectrum is similar to that of yttrium. Neodymium Oxide (NdO); Manganous Oxide (MnO). (Curve a, NdO; curve b, MnO; fig. 86.) The neodymium oxide was deposited in a thick layer upon a strip of platinum, by evaporation from a solution of the nitrate. The radiation curve shows maxima at 3, 4.4, and 4.83 ll. Beyond 6 ll the emissivity is strong and not unlike that of c